Release date: 2014-06-25 Recently, a new electronic "nose" can smell lung cancer. This breath measurement test device contains a chip that distinguishes between normal tissue and cancer tissue, and even gives a stage for cancer. The mortality rate of lung cancer is higher than that of colon cancer, breast cancer and pancreatic cancer. This is because lung cancer is often difficult to detect early, and many of them are diagnosed at an advanced stage. The device is currently being tested by cancer researchers in countries such as the United Kingdom, the United States and Israel, and is expected to reverse this trend, making lung cancer easy to detect early. Scientists presented their results at the American Clinical Oncology Conference in Chicago, where 358 patients were involved in lung cancer or were at risk for lung cancer. A device named NoNose is able to analyze a patient's breath sample to determine if it is suffering from lung cancer. Professor Hossam Haick, inventor of the Israel Institute of Science and Technology, said: "Lung cancer is a catastrophic disease, and the number of deaths due to lung cancer accounts for almost one-third of all cancer deaths. The diagnosis of lung cancer requires invasive tests such as bronchi. Mirror and computer-guided biopsy or surgery. Our equipment integrates new technologies and uses exhaled gases to detect and diagnose lung cancer." Lung cancer tumors produce substances called volatile organic compounds (VOCs) that are easily volatilized into the air while producing a identifiable odor. Professor Haick's use of nanotechnology to develop highly sensitive NaNose chips enables the detection of unique signature VOCs in exhaled gases. NaNose is able to distinguish between 80% of benign and malignant tumors and even distinguish cancer subtypes. Alpha Szenszor of Boston has obtained a technology license and is expected to apply it to the clinic in the next few years. Source: Kexun Medical Network Instrument Ultrasound Therapy Device,Ecg Machine,Ecg Machine 12 Channel,Electrocardiograph Machine 6 Channel,Three Channel Ecg Machine Guangzhou Sonostar Technologies Co., Limited , https://www.sonoeye.com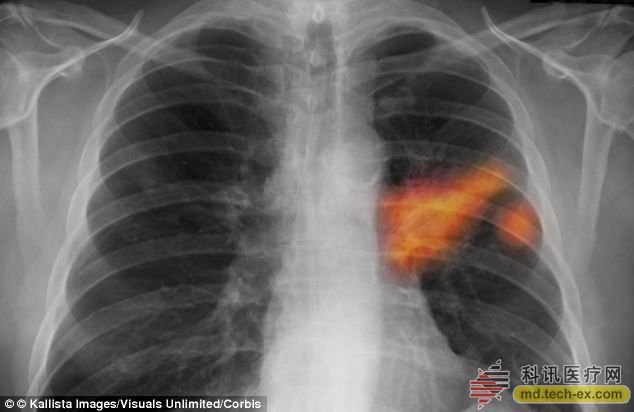
Imaging mode: 1-2-3D, real time display
Locating system: Three dimensional motor platform
Scanning mode: 2Dgalvanometric scanning system
Lateral resolution: 3.8 um
Axial resolution: 4I um
Image depth: 0-2 mm
Speed: 1-50 frame/s
Clinical PW A detection
Port wine stains ( PWS ) is always a birthmark . It is caused by a vascular anomaly. PA imaging could provide real-time information about the thickness of the skin and diameter. depth, distribution of blood vessel , which provide significant help with clinical diagnosis and treatment.
PAI VS. OCT
Image depth : PA 2 mm
OCT 0.4 mm
Functional identification : With optical fingerprint characteristics ,P A could specificity imaging blood vessels , eliminates the interference of hair follicle, sebaceous glands.
The clinical testing has been carried out in 301 and guangzhou military general hospital , testing and statistic all kinds of PWS lesions in patients with vasculal parameters , in order to establish the classification and quantitative treatment of PWS.
New electronic "nose" can diagnose lung cancer early
