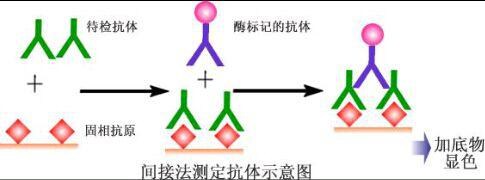
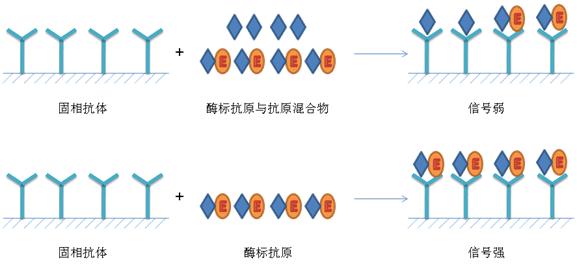
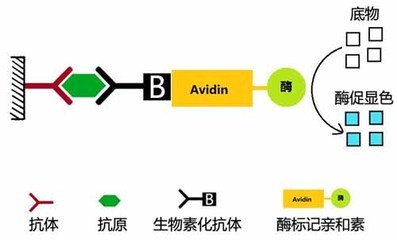
Principle of ELISA The basis of ELISA is the immobilization of antigens or antibodies and the enzymatic labeling of antigens or antibodies. The antigen or antibody bound to the surface of the solid support retains its immunological activity, and the enzyme-labeled antigen or antibody retains both its immunological activity and the activity of the enzyme. At the time of the assay, the test specimen (the antibody or antigen in which it is measured) reacts with the antigen or antibody on the surface of the solid phase carrier. The antigen-antibody complex formed on the solid support is separated from other substances in the liquid by washing. Further, an enzyme-labeled antigen or antibody is added, and is also bound to the solid phase carrier by a reaction. At this time, the amount of enzyme on the solid phase is proportional to the amount of the substance to be tested in the specimen. After the substrate of the enzyme reaction is added, the substrate is catalyzed by the enzyme to become a colored product, and the amount of the product is directly related to the amount of the test substance in the sample, so that qualitative or quantitative analysis can be performed according to the depth of the color. Due to the high catalytic efficiency of the enzyme, the result of the immune reaction is indirectly amplified, and the measurement method achieves high sensitivity. Type of ELISA ELISA can be used to determine antigens and can also be used to determine antibodies. There are three essential reagents in this assay: (1) a solid phase antibiotic pro- or antibody, ie an "immunosorbent"; (2) an enzyme-labeled antigen or antibody, referred to as a "conjugate"; (3) Substrate for the enzyme reaction. Depending on the source of the reagent and the condition of the specimen and the specific conditions of the assay, various types of assays can be devised. There are several types of ELISAs for clinical testing: 夹 Sandwich method (double antibody sandwich method for antigen detection, double antigen sandwich assay antibody) 间接 Indirect method for measuring antibodies (usually used) √ Competition Law (competition method for measuring antigen, competitive method for measuring antibodies) æ•èŽ· Capture coated antibody 亲 Avidin- biotin ELISA 1. Double antibody sandwich assay for antigen The double antibody sandwich method is the most commonly used method for detecting antigens. The steps are as follows: 1) A specific antibody is linked to a solid phase carrier to form a solid phase antibody. Washing removes unbound antibodies and impurities. 2) Add the sample to be tested and keep the reaction. The antigen in the specimen binds to the solid phase antibody to form a solid phase antigen-antibody complex. Wash to remove other unbound material. 3) Add the enzyme-labeled antibody and incubate the reaction. The antigen on the solid phase immune complex binds to the enzyme-labeled antibody. Unbound enzyme-labeled antibody was thoroughly washed. The amount of enzyme carried on the solid support at this time is related to the amount of the antigen to be tested in the specimen. 4) Add substrate to develop color. The enzyme on the solid phase catalyzes the substrate to become a colored product. The amount of antigen in the specimen is measured by colorimetry. In clinical tests, this method is applicable to the determination of macromolecular antigens such as HBsAg, HBeAg, AFP, hCG, etc., of various proteins such as divalent or bivalent. This method can be established by coating a solid phase carrier and preparing an enzyme conjugate as long as a specific antibody against the test antigen is obtained. However, it is not suitable for the determination of haptens and small molecule monovalent antigens (molecular weight less than 5000), because it can not form a two-point sandwich. For example, if the source of the antibody is an antiserum, the antibodies for coating and enzymatic labeling are preferably taken from animals of different species. If monoclonal antibodies are used, two monoclonal antibodies directed against different determinants of the antigen are typically selected for coating the solid support and preparing the enzyme conjugate, respectively. The two-site sandwich method has high specificity, and the test specimen and the enzyme-labeled antibody can be incubated together for one-step detection. In the one-step assay, when the content of the test antigen in the specimen is high, the excess antigen is combined with the solid phase antibody and the enzyme-labeled antibody, respectively, and no "sandwich complex" is formed. Similar to the post-band phenomenon of excess antigen in the precipitation reaction, the absorbance of the color developed after the reaction (located on the excess of the antigen) is the same as the absorbance of the standard curve (located on the excess band of the antibody), such as Normal reading, the result will be lower than the actual content, this phenomenon is called the hook effect, because the standard curve is hooked and bent after reaching the peak. When the hook effect is severe, the reaction may even show no false negative results. Therefore, when using a one-step reagent to measure substances with abnormally high levels of specimens (such as HBsAg, AFP, and urine hCG in serum), the highest value of the measurable range should be noted. Preparation of such agents with high affinity monoclonal antibodies can attenuate the hook effect. If a plurality of identical determinants, such as the a determinant of HBsAg, are present at different sites of the molecule being tested, the same monoclonal antibody for this determination can also be used to coat the solid phase and prepare the enzyme conjugate, respectively. However, in the detection of HBsAg, attention should be paid to the subtype problem. HBsAg has four subtypes of adr, adw, ayr, and ayw. Although each subtype has the same reactivity of a determinant, this is also the use of monoclonal antibody as a sandwich method. Attention to the problem. 2. Double antigen sandwich assay antibody The reaction pattern is similar to the double antibody sandwich method. The specific antigen is coated and the enzyme conjugate is prepared to detect the corresponding antibody. The difference from the indirect method for measuring antibodies is to replace the enzyme-labeled anti-antibody with an enzyme-labeled antigen. In this method, the specimen to be inspected does not need to be diluted, and can be directly used for measurement, so its sensitivity is relatively higher than the indirect method. This method is often used for the detection of anti-HBs in hepatitis B markers. The key to this method lies in the preparation of the enzyme-labeled antigen, and the appropriate labeling method should be found according to the difference in antigen structure. 3. Indirect method for measuring antibodies The indirect method is a commonly used method for detecting antibodies. The principle is to use an enzyme-labeled anti-antibody (anti-human immunoglobulin antibody) to detect a test antibody that binds to a solid phase antigen, so it is called an indirect method. The steps are as follows: 1) A specific antigen is linked to a solid phase carrier to form a solid phase antigen. Washing removes unbound antigen and impurities. 2) Add diluted serum to the test and incubate the reaction. The specific antibody in the serum binds to the solid phase antigen to form a solid phase antigen-antibody complex. After washing, only specific antibodies are left on the solid support, and other components in the serum are washed away during the washing process. 3) Add enzyme-labeled anti-antibody. The total antibody can be detected by enzyme-labeled anti-human Ig, but IgG antibodies are generally detected by using an enzyme-labeled anti-human IgG. The antibody in the solid phase immune complex binds to the enzyme-labeled antibody, thereby indirectly labeling the enzyme. After washing, the amount of enzyme on the solid support is positively correlated with the amount of antibody tested in the sample. 4) Adding substrate color This method is mainly used for the diagnosis of infectious diseases for the detection of pathogen antibodies. The advantage of the indirect method is that a method for detecting the corresponding antibody can be established by using the same enzyme-labeled anti-antibody as long as the transformation is coated with the antigen. The key to the success of indirect methods is the purity of the antigen. Although practical results can be obtained by coating with crude antigens, they should be purified as much as possible to increase the specificity of the assay. Special attention should be paid to the removal of impurities that can react with the serum of normal healthy humans, such as recombinant antigens with E. coli as an engineered enzyme, such as E. coli, which is likely to be resistant to E in blood sputum infected with E. coli. The .Coli antibody reacts. The antigen also does not contain a substance that reacts with the enzyme-labeled anti-human Ig, such as an antigen derived from human plasma or human tissue. If the Ig is not removed, a false-positive reaction also occurs in the test. In addition, if the antigen contains an unrelated protein, it will also affect the coating effect due to competitive adsorption. Another interfering factor in the indirect method is the high concentration of non-specificity contained in normal serum. The specific IgG tested in the patient's serum is only a small fraction of the total IgG. The adsorption of IgG is very strong, and non-specific IgG can be directly adsorbed onto the solid phase carrier, and sometimes it can be adsorbed onto the surface coated with the antigen. Thus, in the indirect method, antigen coating is typically re-coated with an unrelated protein (eg, bovine serum albumin ) to block the free space on the solid phase. In addition, specimens should be diluted first (1:40~1:200) during the test to avoid excessively high negative background results. 4. Competitive test antibody Specific antibodies can be detected by this method when interfering substances in the antigen material are not easily removed, or when it is difficult to obtain sufficient purified antigen. The principle is that the antibody in the specimen competes with a certain amount of the enzyme-labeled antibody for binding to the solid phase antigen. The more the amount of antibody in the specimen, the less the enzyme-labeled antibody bound to the solid phase, so the positive reaction was lighter than the negative reaction. If the antigen is of high purity, it can be coated directly with the solid phase. If there is an interfering substance in the antigen, the direct coating is not easy to be successful, and the capture coating method may be employed, that is, the antibody corresponding to the solid phase antigen is first coated, and then the antigen is added to form a solid phase antigen. The impurities in the antigen are washed and removed, and then the specimen and the enzyme-labeled antibody are added for competitive binding reaction. Competitive assays have a variety of antibodies that allow for the binding of specimens and enzyme-labeled antibodies to solid-phase antigens. This is commonly used in anti-HBc ELISAs. Another mode is to add the specimen together with the antigen to the solid phase antibody for competitive binding, and then add the enzyme-labeled antibody after washing to react with the antigen bound to the solid phase. This method is generally used for the detection of anti-HBe. 5. Competition method for measuring antigen Small molecule antigens or semi-antibiotics lack two or more sites that can be used as sandwich methods, and therefore cannot be measured by the double antibody sandwich method, and a competition method mode can be employed. The principle is that the antigen in the specimen competes with a certain amount of the enzyme-labeled antigen for binding to the solid phase antibody. The more the antigen content in the specimen, the less the enzyme-labeled antigen bound to the solid phase, and the final color development is shallower. This method is often used for ELISA assays such as small molecule hormones and drugs. 6. Capture coated antibody Detection of IgM antibodies is used in the early diagnosis of infectious diseases. Indirect ELISA is generally only suitable for detecting total antibodies or IgG antibodies. If the IgM antibody is directly assayed by indirect method of antigen coating, a higher concentration of IgG antibody is generally present in the sample, and the latter will compete for binding to the solid phase antigen so that a part of the IgM antibody cannot bind to the solid phase. Therefore, if IgM antibodies are indirectly measured using anti-human IgM as a secondary antibody, the sample must first be treated with protein A or anti-IgG to remove IgG interference. The capture coating method is often used in the determination of antibody IgM in clinical tests. The solid phase is first coated with an anti-human IgM antibody to capture IgM in serum samples (including specific IgM antibodies to the antigen and non-specific IgM). The antigen is then added and this antigen binds only to specific IgM. The enzyme is then labeled with an antibody specific for the antigen. Then with the substrate, the coloration is positively correlated with the IgM in the specimen. This method is often used for early diagnosis of viral infections. Rheumatoid factor (RF) can also interfere with the capture coating assay for IgM antibodies, leading to false positive reactions. Therefore, indirect methods of neutralizing IgG have recently been favored, and detection of anti-CMV IgGM and anti-Toxoplasma IgM antibodies with such agents has been successful. 7. ABS-ELISA ABS is an abbreviation of avidin biotin system. Avidin is a glycoprotein with a molecular weight of 60,000 and consists of four subunits that bind to biotin. Biotin is a small molecule compound with a molecular weight of 244. The chemically-derived derivative-hydroxysuccinimide ester forms a biotin-labeled product with various types of large and small molecules such as proteins and sugars, and the labeling method is quite simple. The binding of biotin to avidin is highly specific, and its affinity is much greater than that of antigen-antibody, and the two are extremely stable upon combination. Since one avidin can bind to four biotin molecules, the ABS and ELISA methods can be divided into enzyme-labeled avidin-biotin (LAB) method and bridged avidin-biotin (ABC) method. Types. Both replace the enzyme-labeled antibody (antigen) in the original ELISA system with a biotinylated antibody (or antigen). In LAB, solid phase biotin is first reacted with unlabeled avidin followed by enzyme-labeled biotin to further increase sensitivity. In the early stage, avidin was extracted from egg white. This egg avidin is a basic glycoprotein, which has strong adsorption with a polystyrene carrier and can be used in ELISA to increase the background. Streptavidin extracted from Streptomyces does not have this disadvantage, and there is a tendency to replace the former in ELISA applications. Because ABS-ELISA uses two reagents more than ordinary ELISA, the operation steps are increased, and ABS-ELISA is not used much in clinical tests. PETG Orthopedic Implants Packaging
Aseptic medical plastic blister trays. Producet in clean room. Special for medical device Packaging.
light weight, convenient transportation, good sealing performance, in line with the requirements of environmental protection and green packaging; Can pack any special-shaped products, packing without additional cushioning materials; The packaged products are transparent and visible, beautiful in appearance, easy to sell, and suitable for mechanization, automatic packaging, convenient for modern management, labor saving, improve efficiency
Petg Blister,Sterile Implant Packaging,Orthopedic Implants Packaging,Disposable Medical Plastic Trays taicang hexiang packaging material co.,ltd , https://www.medpackhexiang.com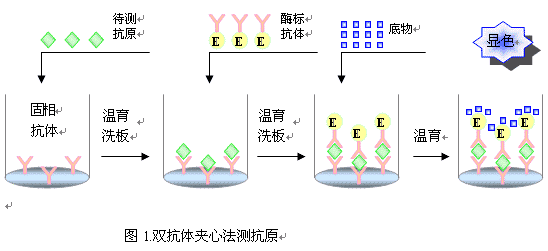
Principle and type of ELISA experiment
Related Technical Services: ELISA
