The development of mobile medical care will become increasingly vigorous. In order to open up new blue ocean business opportunities, manufacturers of traditional medical equipment and information communication (ICT) products are all actively moving towards the mobile medical market. For example, the development of wearable medical devices and portable ultrasonic devices will not only make the mobile medical ecosystem more complete. It also drives the needs of microcontrollers (MCUs) and various wireless communication semiconductor components. Mobile medical business opportunities are continuing to emerge in national markets. Especially in advanced countries such as Europe, America, and Japan, due to the serious population aging problem, the relevant government and health management agencies have spared no effort to promote mobile medical services, thereby reducing the huge medical expenses to reduce the national financial burden. For the card mobile medical market, in the first half of this year, many medical products or service providers launched a merger and acquisition campaign to enhance mobile medical device or application development capabilities, including cloud electronic medical record service and software developer Athenahealth in January this year to 2.93 Billion dollars bought Epocrate, a mobile medical application developer; and wearable health product supplier Jawbone acquired MassiveHealth, Visere and BodyMedia to enhance the lineup of physiological information monitoring technology and application products. In addition, ICT operators are also eager to test mobile medicine. For example, Microsoft, Intel, Samsung and Qualcomm have joined the Continua Alliance to actively promote the industry's common standards and have recently offered new ones. Product development strategy. For example, QualcommLife bought HealthyCircles in May to improve the software-as-a-service (SaaS) operation mode of its 2net platform. Microsoft plans to deploy mobile medical information management services on the Bing platform. As for Intel and Samsung, they continue to increase their investment. And expand cooperation with medical equipment factory, in the development of the mobile medical industry to pay attention to strong momentum. However, with the accelerated development of mobile medical care, the US Food and Drug Administration (FDA) also plans to set the mobile medical application (MMA) specification in the fourth quarter of this year to guide the mobile medical care industry to gradually lead to the boundary between medical and health care behavior. Awkward chaos. New FDA regulations will face new changes in mobile medical care The US FDA introduced the MMA guidelines as early as 2011. In 2012, it included more definitions and regulations, and revealed that the final specifications will be announced before the end of 2013. At the same time, the pre-market certification operations will be initiated to ensure the safety and functionality of mobile medical software and hardware. The supply chain operators have been driven to prepare for the war. Figure 1 Huang Yubin, an analyst at the IKK Medical Equipment and Health Care Research Department of the ITRI, said that Taiwan's ICT industry must enter the mobile medical industry and pay close attention to the FDA's regulatory trends. Lithium Carbonate CAS No.554-13-2 Lithium Carbonate Basic Information
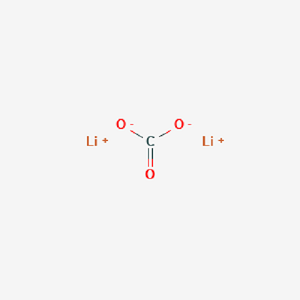
Lithium Carbonate Structure
Lithium Carbonate,Lithium Carbonate Dosage,Uses For Lithium Carbonate,Lithium Carbonate Uses,Lithium Carbonate Side Effects ShanDong YingLang Chemical Co.,LTD , https://www.sdylhgtrade.com
CAS: 554-13-2
MF: CLi2O3
MW: 73.89
EINECS: 209-062-5
Mol File: 554-13-2.mol
Melting point 720 °C
Boiling point 1342 °C(lit.)
density 2.11 g/mL at 25 °C
Fp 1310°C
storage temp. Store at +5°C to +30°C.
solubility 13g/l
form wire
Specific Gravity 2.11
color White
PH 10-11 (5g/l, H2O, 20℃)
Water Solubility 13 g/L (20 ºC)
Medical/ICT players begin to compete in the mobile medical market
