Release date: 2014-12-25 The EU first approved a stem cell therapy that can be used clinically within the EU. This stem cell therapy is used to treat an eye condition that can lead to blindness. This treatment, called Holoclar, is reported to be effective in cases where 80% of limbal stem cells are deficient. The European Medicines Agency said the decision was a major development in the field of stem cell therapy. Defects in limbal stem cells can lead to a series of eye lesions, such as scarring on the ocular surface, which eventually leads to blindness. Research clinics and some specialized centers have been clinically testing Holoclar for the replacement of limbal stem cells. The decision of the European Medicines Agency means that this therapy can now be applied to a wider range of clinical and medical treatments without being limited to a small range of studies. (Original title: EU first approved a wide-ranging clinical use of stem cell therapy) Source: China News Network Shanghai J.Shine Co.,Ltd , https://www.jshinechem.com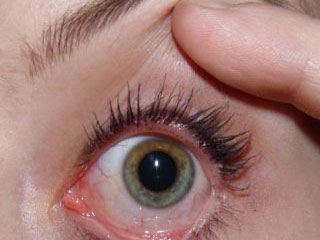
The EU first approved a wide-ranging clinical use of stem cell therapy
The treatment known as Holoclar is effective in cases where 80% of limbal stem cells are deficient, and this therapy is approved for clinical use by the European Union.
