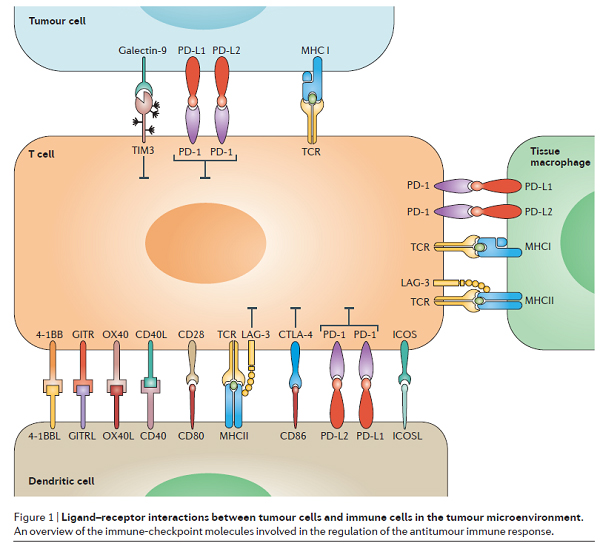
As a key component of cancer immunotherapy, immunological checkpoint blocking therapies (such as PD-1/PD-L1 antibodies) have subverted the treatment of a variety of advanced cancers. However, at present, only a small number of patients are able to respond to such therapies. Scientists have been trying to develop biomarkers that can predict this group of responders. Recently, a review on Nature has been divided into 7 categories, making the most comprehensive inventory of biomarkers that can predict the response of immunotherapy. As a key component of cancer immunotherapy, immune-checkpoint blockade (ICB) therapy subverts the treatment of a variety of advanced cancers. Monoclonal antibodies targeting the immunological checkpoint proteins CTLA-4, PD-1/PD-L1 have been approved for the treatment of various types of malignancies. However, it cannot be ignored that at present, only a small number of patients are able to respond to this revolutionary therapy. At present, there are two popular research directions in the field of immunotherapy. On the one hand, scientists are trying to find ways to expand the response population so that more patients can benefit from such treatments; on the other hand, they are also actively studying the ability to predict the patient's ability to respond to immune checkpoint inhibitors. Biomarkers, hope to achieve accurate immunotherapy. On June 27th, four scientists from the Dana–Farber Cancer Institute in the United States published a review entitled “Monitoring immune-checkpoint blockade: response evaluation and biomarker development†on Nature Reviews Clinical Oncology. The article summarizes the various strategies for assessing patient response and the latest advances in biomarker research. The authors hope to maximize the benefits of immune checkpoint blockade therapy. cutting edge Immune checkpoint blocking therapy exerts an anticancer effect by activating an antitumor immune response. Among them, T cells play an important role in the immune defense mechanism against cancer: they recognize tumor antigens, then are activated, spread, and eventually destroy cancer cells. In fact, activation of T cells is regulated by ligand-receptor interactions on macrophages in T cells, dendritic cells, tumor cells, and tumor microenvironment (TME). These receptor-ligand pairs are called immunological checkpoints. Figure 1 summarizes the immunological checkpoint molecules involved in the regulation of anti-tumor immune responses. Image source: Nature Reviews Clinical Oncology At present, the US FDA has approved a number of monoclonal antibodies targeting CTLA-4, PD-1, and PD-L1 immunological checkpoints. The authors believe that the scope of application of immunological checkpoint blockade therapy is rapidly expanding in clinical oncology practice. This trend will continue to grow with the approval of new drugs. Therefore, a comprehensive understanding of the benefits and risks associated with such therapies is critical to the patient. Treat Pharyngeal Extract,Therapeutic Pharyngeal Extract,Red Beet Extract Betanin Powder,Plant Extract Licorice Root Powder Shaanxi Zhongyi Kangjian Biotechnology Co.,Ltd , https://www.zhongyiherbs.com
Nature teaches you how to move towards the era of precision immunotherapy
