Cam Locks,Door Cam Lock,Window Cam Lock,Drawer Cam Lock ZHEJIANG ZHONGZHENG LOCK INDUSTRY CO.,LTD , https://www.zhongzhengcabinetlock.com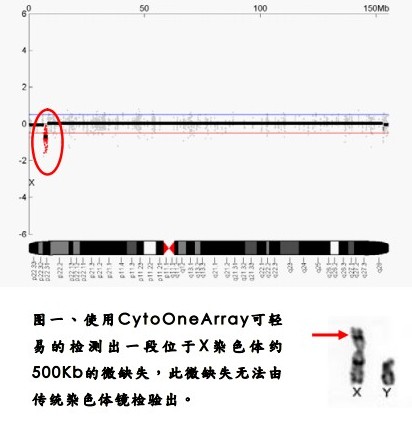
Chromosome chip for prenatal examination
2012.12. A clinical study published by the New England Journal of Medicine at Columbia University Medical Center shows that Chromosomal microarray provides more clinical information than traditional karyotyping and can be used for prenatal diagnosis. Standard practice. Hualian Biotech “Phalanx Biotech Group†was launched in 2012. 05. The chromosome chip “CytoOneArray†is a product designed for clinical molecular/genetic testing.
Current prenatal testing standards for current prenatal testing standards
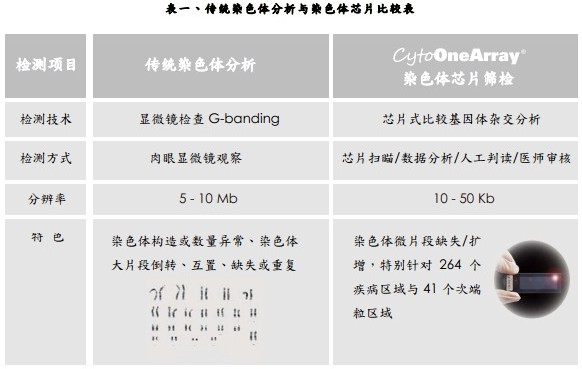
Chromosome karyotyping has been the standard practice of prenatal testing since 40 years ago. It mainly detects abnormalities such as chromosome structure, number and large fragments of chromosomes, such as inversion, interposition, deletion or duplication, but limited by resolution. A small fragment of the chromosome was detected to be missing.
Obstetricians and gynecologists in the prenatal examination of pregnant women, such as the detection of high risk factors for Down's syndrome, or abnormal ultrasound observation, will be performed amnion (villi) puncture, amniotic fluid to obtain fetal cells, amniocytes After culturing, karyotype analysis was performed with a high power microscope after 2 to 3 weeks to see if the fetus had a chromosomal variation.
After the relevant test report is completed, the gynaecologist and the gynaecologist will make a comprehensive judgment to make diagnosis and suggestions for the pregnant woman and its fetus; due to the time pressure during pregnancy, and the knowledge of genetic testing, how to detect it In the information, to determine whether the fetus has chromosomal variation, it is imperative to update the better detection tools.
Meet the CytoOne-Array chromosome chip
Hualian Biotech uses the array CGH technology to develop the CytoOne-Array chromosome chip, which is different from other scientific research products. It is designed as a tool for clinical chromosomal genetic testing. This clinical test chip can be used to detect micro-fragment deletion diseases. Since the launch of the product/service, clinical case testing services have been carried out, and the service cases have spread throughout the major medical centers in China. The chip probe design is a chromosome chip product designed for a specific 264 disease region and 41 subtelomeric regions known. The determination of the abnormal signal is calculated by numerical calculation, and it is not dependent on the naked eye, so the resolution can reach 10-30 kb. Compared with the 5 Mb size of the traditional chromosome mirror, more nuances can be found (Fig. 1).
In non-specific areas, probes with a resolution of 2 Mb are also designed and avoid common copy number variation segments (Copy
Number variation, CNV). The chromosomal micro-deletion or micro-duplication abnormalities in these 264 disease areas are supported by clinically published evidence, and most of the abnormalities in chromosome deletion or amplification in non-disease areas are Larger fragments will cause illness. Such a design can provide a clearer interpretation by the clinician while still maintaining the ability to detect in non-disease areas.
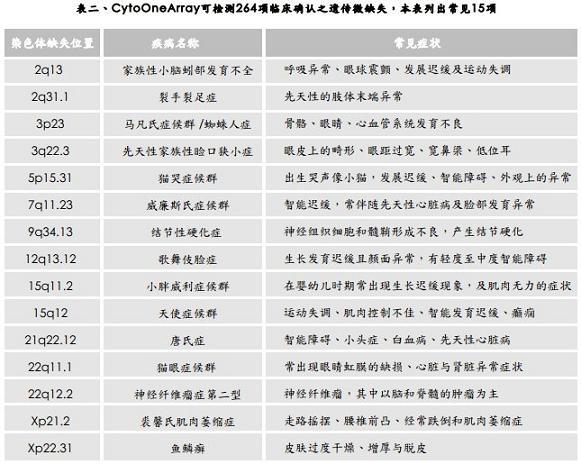
CytoOneArray® products are particularly concerned with microdeletions and amplification associated with stunting symptoms in specific diseases. After careful clinical literature review, CytoOneArray® products have clinical literature on chromosome segments associated with specific diseases. See the disease (Table 2).
Recommended object to be tested
Pregnant women with the following conditions are more likely to perform chromosome chip screening:
1. Older women over 34 years old
2. High risk group after Down's screening
3. Ultrasound examination shows abnormal signs in the fetus
4. Infants who have previously developed Down's syndrome or other abnormal infections
5. Parents with chromosomal abnormalities
Screening processes
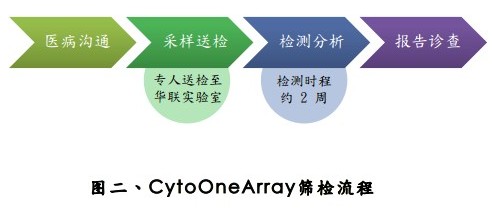
Pregnant women who meet the recommended target, after the explanation by the professional gynaecologist or consultant, fill out the "CytoOneArray Chromosome Chip Test Consent", and then take 10~15c.c. when performing amniocentesis or villus puncture. The sample is sent to the Hualian Genome Laboratory by a special person. A report can be issued in about 2 weeks, and the test result is reported by the doctor.
summary
Even though the literature of Columbia University pointed out that chromosome chips can provide more clinical information than traditional karyotype analysis, it can be a standard tool for prenatal diagnosis. The same journal has a more neutral opinion on this article. When the CNV detected by the chromosome chip is still clinically uncertain, it cannot be judged whether it will cause fetal genetic abnormality, which may cause some ethical problems; in view of this, Hualian chromosome The chip is specifically interpreted for disease areas identified in the literature, and the geneticist carefully explains whether it is related to the occurrence of the disease, and finally reports to the subject. Hualian treats every case with caution.
references
(1) Ronald J. Waner et. al. Chromosomal Microarray versus Karyotyping for Prenatal Diagnosis. The New England Journal of Medicine (2012) vol. 367, No.23 : 2175-2184
(2) Lorraine Dugoff. Application of Genomic Technology in Prenatal Diagnosis. The New England Journal of Medicine (2012) vol. 367, No.23 : 2249 - 2251
(3) George McGillivray et. al. Genetic counselling and ethical issues with chromosome microarray analysis in prenatal testing. Prenatal Diagnosis (2012), 32, 389–395
(4) Justin Petrone. NEJM Papers Support Use of Arrays inPrenatal Dx as Sequencing Gains Ground. GenomeWeb (2012) December 11
