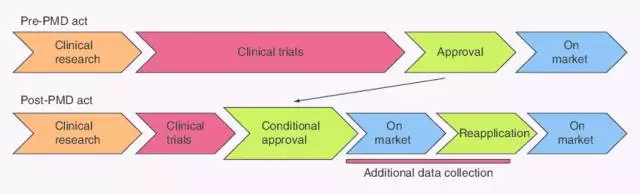
Japan is expected to become a global R&D base for regenerative medicine . The new law implemented in Japan in the fall of 2014 has greatly shortened the time for approval of regenerative medicines, making Japan the first country in the world to put scientific research into practical use. Attracted by this, in addition to Japanese companies, overseas bio-startups have also entered Japan. In the past, Japan was taken away because of the complicated procedures for the approval of drugs. Today, the situation has changed. Regenerative medicine refers to the use of biological and engineering theories to create tissues and organs that are lost or functionally damaged, and that have the mechanisms and functions of normal tissues and organs. Broadly speaking, regenerative medicine originally refers to the theory, technology, and surgical operations of tissue regeneration in the body. It can also be understood as an effective biological treatment method by studying the normal tissue characteristics and functions of the body, the mechanism of wound repair and regeneration, and the mechanism of stem cell differentiation. To promote the body's self-repair and regeneration, or to construct new tissues and organs to maintain, repair, regenerate or improve damaged tissue and organ function. In the narrow sense, it is the application of the principles and methods of life sciences, materials science and other disciplines, research and development of new disciplines and cutting-edge cross-cutting fields for the theory and technology of replacing, repairing, reconstructing or regenerating various tissues and organs of the human body. In 2014, Japan passed the The Pharmaceuticals and Medical Devices Act (PMD Act), which specifically awards a new green channel for cell therapy in the field of regenerative medicine: as long as it proves the safety and predictable efficacy of the product, The conditions approved by the competent authority can be obtained to promote the sale of the product and the subsequent evaluation of the efficacy of the clinical trial. (expedited approval system) Japan implemented the Pharmaceutical Medical Devices Act in November of the same year, and the approval period for regenerative medical drugs has been significantly shortened from the previous seven years. It will be available in the next 2 to 3 years. This speed is even ahead of Europe and the United States. Pluristem Therapeutics Vaseline Gauze,Vaseline Gauze Pads,Vaseline Coated Gauze,Sterile Vaseline Gauze Henan Anbang Medical Supplies Co., Ltd. , https://www.anbangmedical.com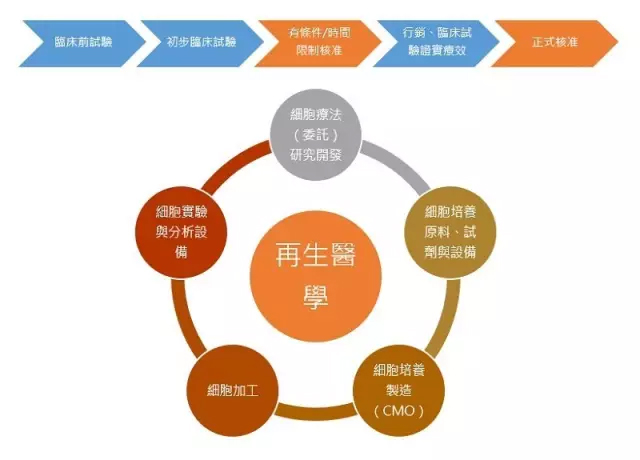

The global regenerative medical market reaches nearly 10 billion US dollars: Japan is expected to become a research and development base
