The General Office of the Food and Drug Administration has publicly requested the abolition of 26 medical device industry standard opinions such as YY 0097-1992 "Magnetic Health Cup" In accordance with the relevant requirements of the "Notice of the State Council on Printing and Deepening the Standardization Work Reform Plan" (Guo Fa [2015] No. 13), the State Food and Drug Administration intends to abolish YY 0097-1992 "Magnetic" through the integration and streamlined review of medical device industry standards. 26 medical device industry standards such as the Health Care Cup (see attachment) are now open for comments. Please send feedback by email before June 30, 2017 (please specify the subject: XXX units to comment on the abolition of industry standards). Attachment: List of medical device industry standards to be abolished Importance: 1. Bottle should be used within 60 days of first opening.
2. Keep away from light and moisture.
3. Do not leave in the bathroom to avoid moisture.
4. Tightly replace cap after removing strips from the bottle.
5. Do not remove desiccants or touch test areas.
6. Use within expiration date.
Reading the results:
Negative: reduce carbohydrates intake or increase excercise.
Trace/ small/ moderate: your health is satisfactory and you are burning fat.
Large: increase carbohydrates or reduce exercise. Urine Test Strips Cvs,Home Urine Test Strips,Negative Uti Test Strip,Urine Test Strips For Ovulation Changchun LYZ Technology Co., Ltd , https://www.lyzinstruments.com
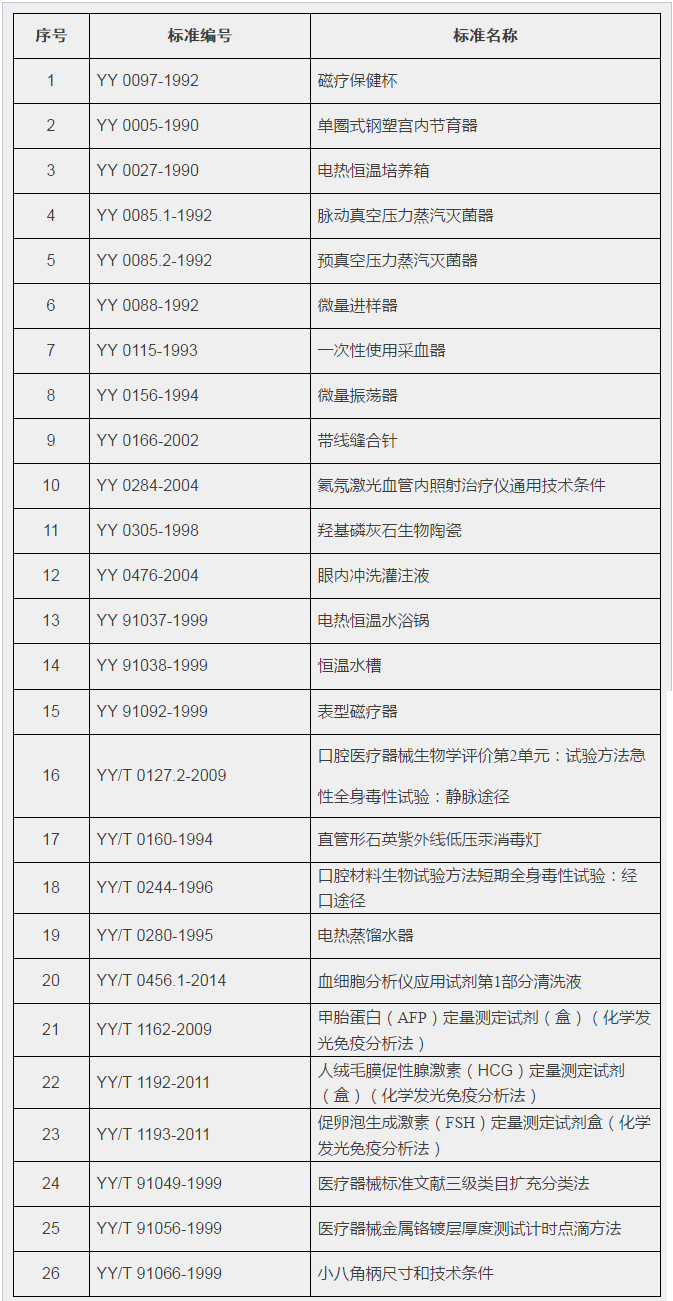
CFDA plans to abolish 26 medical device industry standards
