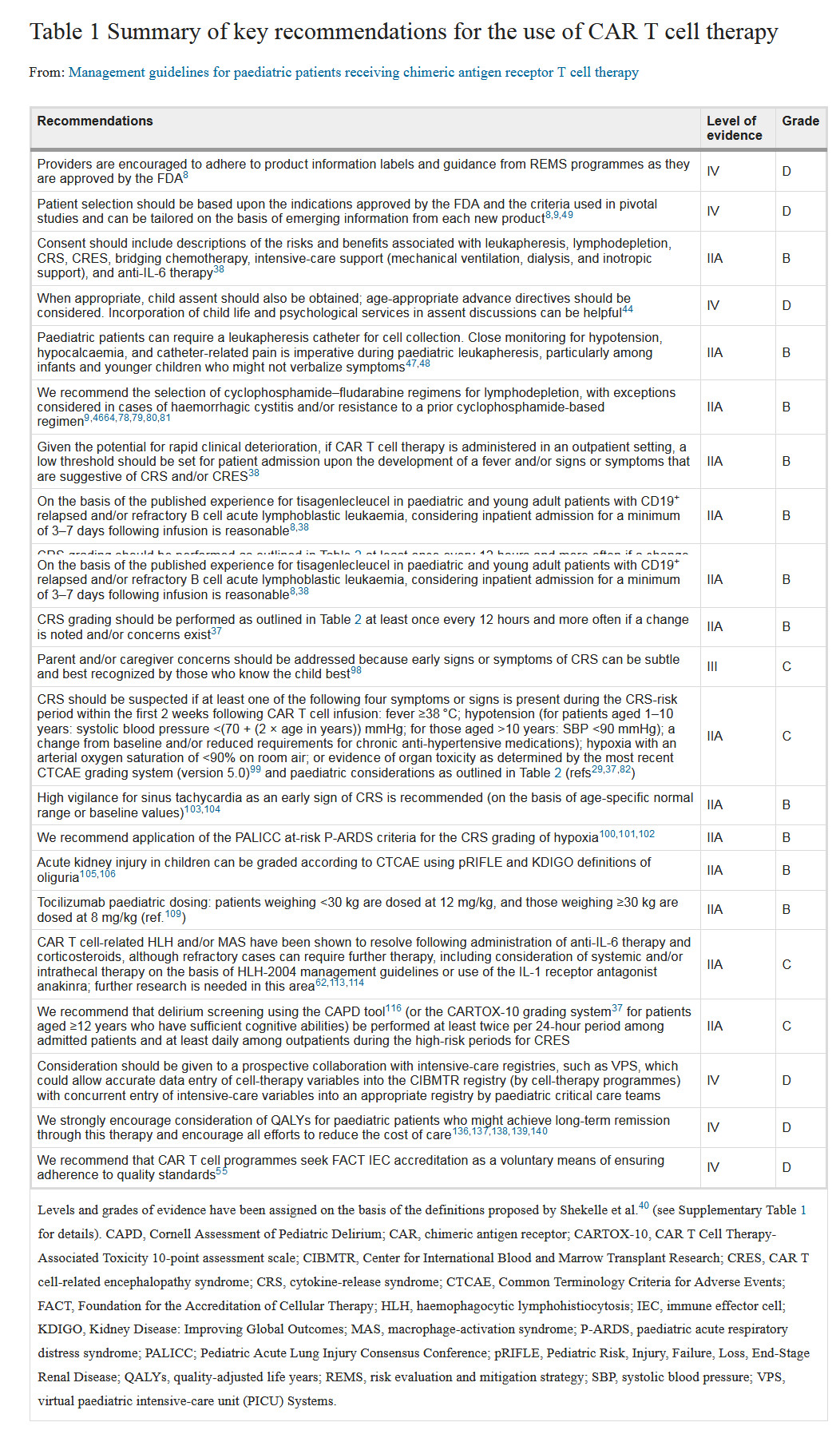
The Nature Supplement publishes a guide to CAR-T therapy! Associated childhood acute lymphoblastic leukemia August 08, 2018 Source: Biological Exploration Editor's Note: Last August, the FDA approved CAR-T therapy for the treatment of acute lymphoblastic leukemia (ALL). A year later, scientists at the University of Texas MD Anderson Cancer Center, in conjunction with the Pediatric Acute Lung Injury and Sepsis Researcher Network (PALISI), published a comprehensive consensus guide on children receiving CAR-T cell therapy. The guide brings together the lessons learned by top experts in identifying early signs and symptoms of treatment-related toxicity and details how to respond to these symptoms. On August 6, "Nature Reviews Clinical Oncology" published this guide online with the topic "Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy". Acute lymphoblastic leukemia (ALL) is a neoplastic disease originating from B-lineage or T-lineage lymphoid progenitor cells. The primordial cells proliferate and aggregate in the bone marrow and inhibit normal hematopoiesis, leading to anemia, thrombocytopenia and neutrality. Granulocyte reduction; primordial cells can also invade extramedullary tissues, such as meninges, gonads, thymus, liver, spleen, or lymph nodes, causing corresponding lesions. CAR-T therapy is a genetic modification of a specific protein (chimeric antigen receptor, CAR) into a T cell after extraction of a patient's T cells. After reinjection of the modified T cells into the human body, the antigen (CD19) on the surface of leukemia cells can be specifically identified and killed. The data showed that the success rate of ALL patients after CAR-T clinical cell immunotherapy reached 90%. The ongoing research is aimed at expanding the use of this therapy in other cancers. Image source: CC0 Creative Commons Although CAR-T therapy has shown great promise, Dr. Kris Mahadeo, MD, associate professor of pediatrics at MD Anderson, said: "CAR-T cell therapy is associated with a significant response rate for children and young people, but this innovative form Cellular immunotherapy is prone to unique and severe toxic reactions that can lead to rapid cardiopulmonary and/or neurological deterioration. This approach also requires a diverse, multidisciplinary team and relevant clinical infrastructure to ensure optimal outcomes. ." Mahadeo added that with the increasing use of CAR-T cell therapies, treatment guidelines, comprehensive training for multidisciplinary staff, and other measures should help to properly manage the toxicity of this new treatment. To this end, MD Anderson's CAR-T Cell Therapy-Related Toxicity (CARTOX) project, in collaboration with PALISI and its Hematopoietic Stem Cell Transplant (HSCT) team, provides a comprehensive guide for children receiving CAR-T cell therapy. The guide brings together experts from a wide range of fields, including pediatric intensive care physicians, pharmacy specialists, neurologists, and translational immunotherapeutics research experts, to provide important knowledge designed to help improve patient outcomes and outcomes. The following is part of the guide: (1) When the Risk Assessment and Mitigation Strategy (REMS) project is approved by the FDA, the supplier is advised to comply with the product information label and guidelines; (2) The recommended patient selection should be based on FDA-approved indications and criteria used in key studies, and can be customized based on new information for each new product; (3) Consent should include leukemia, lymphatic failure, cytokine release syndrome (CRS), CAR-T cell-associated encephalopathy syndrome (CRES), bridging chemotherapy, intensive care support (mechanical ventilation, dialysis, and muscle support) a description of the risks and benefits associated with anti-IL-6 therapy; (4) It is advisable to seek the views of the child, where appropriate; consideration should be given to age-advanced directives, and it may be helpful to include child life and psychological services in the discussion; (5) Pediatric patients may require a leukocyte separation catheter to collect cells. In pediatric leukocyte separation, it is recommended to closely monitor hypotension, hypocalcemia, and catheter-related pain, especially in infants and young children who cannot describe the symptoms; (6) It is recommended that the cyclophosphamide-fludarabine regimen be used to treat lymphatic failure, except for hemorrhagic cystitis and/or previous resistance to cyclophosphamide regimens; (7) Considering the possibility of rapid clinical deterioration, if CAR-T cell therapy is performed in an outpatient setting, it is recommended to set a lower patient for fever and/or suggestive of symptoms of CRS and/or CRES. Entrance threshold; (8) Based on the experience of the published CAR-T drug tisagenelecleucel in pediatric and young adult patients with CD19+ relapsed and/or refractory B-cell acute lymphoblastic leukemia, it is recommended to be hospitalized for at least 3-7 days after infusion; (9) With changes in clinical status, it is recommended to perform CRS and CAR T cell-associated encephalopathy syndrome (CRES) grading every 12 hours or more (outpatient management, including caregivers); (10) Resolve concerns of parents and/or caregivers in a timely manner, as the early signs or symptoms of CRS may be subtle, and the person who knows the child best will know best; (11) It is recommended that within the first two weeks after CAR-T cell injection, if the patient has the following four symptoms or signs, it should be suspected to be cytokine release syndrome CRS: fever ≥ 8 ° C; hypotension (for 1- 10-year-old patients with systolic blood pressure <(70+(2*age)mmHg); for children over 10 years of age, systolic blood pressure below 90 mmHg); baseline changes and/or reduced requirements for chronic antihypertensive drugs; arterial oxygen saturation Less than 90%; or evidence of organ toxicity as defined by the Common Adverse Event Terminology Standards System (CTCAE Version 5.0); (12) It is recommended to be highly alert to sinus tachycardia, an early sign of CRS; (13) It is recommended to use pediatric acute respiratory distress syndrome as a CRS hypoxia grading standard; (14) It is recommended that children with acute kidney injury can be classified according to CTCAE; (15) Recommended dosage of Tocilizumab in children: body weight <30kg, dose 12mg/kg; body weight ≥30 kg, dose 8 mg/kg; (16) CAR-T-associated hemophagocytic reticulosis (HLH) and macrophage activation syndrome (MAS) have been shown to be resolved by anti-IL-6 therapy, corticosteroids; (17) It is recommended to use the Cornell Assessment (CAPD) to screen patients for unconsciousness (or the CARTOX-10 grading system for patients over 12 years of age with adequate cognitive ability) at a frequency of at least twice every 24 hours; (18) It is recommended that the prospective cooperation with the intensive care unit be carried out in order to accurately enter the cell treatment variables into the CIBMTR registry and to enter the intensive care variables into the appropriate registry; (19) Strongly support the consideration of quality-adjusted life years (QALYs) for pediatric patients and strive to reduce the cost of care; (20) It is recommended that CAR-T therapy be based on the Cell Therapy Certification Foundation (FACT) immune effector cell certification as a voluntary means of ensuring compliance with quality standards. Elizabeth Shpall, MD, said: "CARTOX is responsible for overseeing the care of patients with MD Anderson CAR-T cell therapy and is the first independent immune effector cell therapy program certified by the Foundation for the Accreditation of Cellular Therapy (FACT). The project provides oversight of more than 20 active immune effector cell research protocols and two approved care treatment standards at the MD Anderson Center. These new guidelines will undoubtedly become important new models for CAR-T cell patient care." Reference materials: 1)Acute lymphoblastic leukemia: Comprehensive pediatric CAR-T guidelines developed 2) Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy Vaccine For Rabies Prophylaxis Travelers to areas where rabies is endemic may be at risk, especially if they are likely to come in contact with animals in areas where dog or other animal rabies is enzootic and immediate access to appropriate medical care is unlikely. Canine rabies remains highly endemic in certain areas of the world. Need for rabies preexposure vaccination depends on the nature of risk and associated level of potential exposure. preexposure vaccination based on local incidence of rabies in the country to be visited, availability of appropriate agents for rabies postexposure prophylaxis in that country, and intended activity and duration of stay Vaccine For Rabies Prophylaxis,Rabies Prophylaxis Vaccine,Freeze Rabies Prophylaxis Vaccine,Freeze Vaccine For Rabies Prophylaxis Changchun Zhuoyi Biological Co., Ltd , https://www.zhuoyi-bio.com
The Nature Supplement publishes a guide to CAR-T therapy! Associated childhood acute lymphoblastic leukemia
