Jennifer L. Simeone and Paul D. Rainville purpose background By changing the MS inlet technology from RPLC to UPC 2 , it is possible to directly inject polar compounds from highly organic samples without additional sample preparation steps such as evaporation and reconstitution. Figure 1. Example chromatogram of caffeine PPT extract obtained by (a) 1 μL injection, (b) 3 μL injection, and the same coffee in the ACQUITY UPC 2 system in UPLC® operating in reversed mode. An example chromatogram of the (c) 7 μL injection of PPT extract. solution Figure 1 compares the caffeine-containing PPT extract in a 1 μL direct injection and 3 μL direct injection in an ACQUITY UPLC system with a 7 μL injection in the ACQUITY UPC 2 system. The 1 μL injection in RPLC showed a good peak shape, but the peak shape was deformed at 3 μL injection volume, and the forward peak and peak bifurcation were observed. The 7 μL injection using UPC 2 still showed good peak shape. Similar results were observed for other polar molecules tested in the same manner (Table 1). Table 1 also shows the maximum injection volume of all analytes tested in the PPT extract when using RPLC and UPC 2 . These data clearly demonstrate the advantages of using the ACQUITY UPC 2 System to analyze polar compounds in highly organic extracts, and do not require additional sample preparation prior to injection as in the RPLC system, simplifying workflow. to sum up Xray Spine Board,Detached Spine Board,Carbon Fiber Spine Board,Ambulance Transfer Spine Board jiangyin chenyi medical technology co.,ltd , https://www.chenyimed.com
Direct injection of strongly polar compounds in protein-precipitated plasma for bioassay.
Most bioanalytical methods use protein precipitation (PPT) extraction because the technique is simple, fast, and low cost. A typical PPT uses about 35% organic solvent and biological sample to give about 75% organic extract. Traditionally, samples were analyzed using reverse phase liquid chromatography (RPLC). For strongly polar compounds, the chromatographic peak shape due to strong solvent action is poor, the most obvious is peak extension and/or peak bifurcation, so high concentration organic extract cannot be directly injected into the RPLC system. Therefore, additional sample processing, including evaporation and reconstitution or dilution with water, is required prior to injection into the chromatographic system. 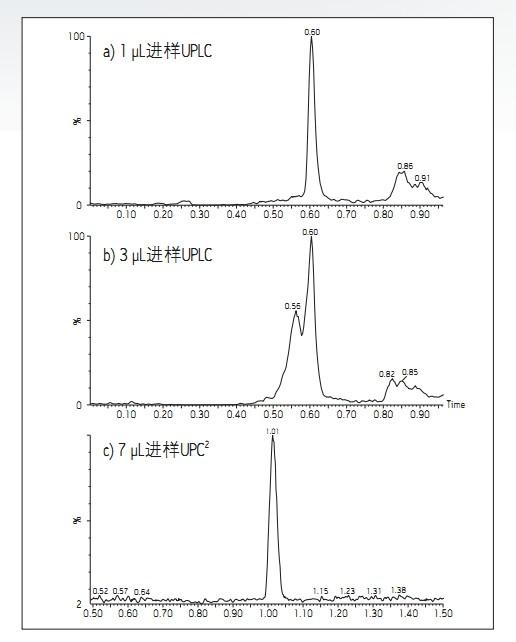
UltraPerformance Convergence ChromatographyTM (UPC 2 ®) uses supercritical carbon dioxide as the primary mobile phase, and its retention mechanism is different from that of RPLC, allowing direct injection of highly organic extracts. In this example, four polar compounds were extracted from rat plasma by PPT extraction using acetonitrile at a ratio of 3:1, injected directly into the ACQUITY UPC 2 TM system, and using a conventional RPLC system as Compared. UPC 2 analysis was performed on an ACQUITY UPC 2 BEH column using ammonium hydroxide modified methanol as the cosolvent (mobile phase B). RPLC analysis was performed on an ACQUITY UPLC® system equipped with an ACQUITY UPLC BEH C 18 column using water and ammonium hydroxide modified acetonitrile as the mobile phase. 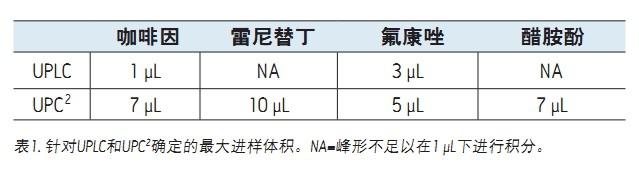
By changing the MS inlet technology from standard RPLC to UPC 2 , polar compounds dissolved in highly organic samples can be injected directly into the ACQUITY UPC 2 system without additional sample preparation steps such as evaporation and reconstitution. Adding UPC 2 to bioanalytical laboratories simplifies the analysis of polar compounds in highly organic sample preparations.
Direct injection of polar compounds in protein-precipitated plasma using UPC2/MS/MS
