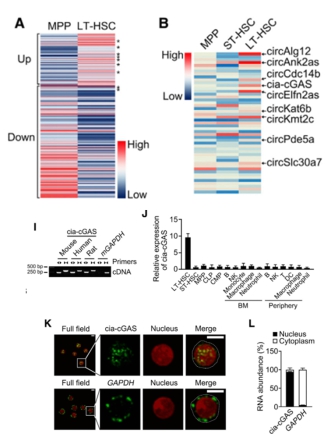
Prof. Fan Zusen from the Institute of Biophysics of the Chinese Academy of Sciences is mainly engaged in the research of cancer stem cells, immune cell development and differentiation, tumor target discovery and individualized treatment of tumors. Recently, his team used Arraystar Mouse CircRNA Array to study mouse bone marrow cells (BM). Expression profiles of circRNAs isolated from long-term hematopoietic stem cells (LT-HSCs) and pluripotent stem cells (MPPs). The study screened a circRNA cia-cGAS with high expression in the nucleus of LT-HSCs. Through a series of functional mechanisms, it was found that cia-cGAS in LT-HSCs can bind to DNA-sensitive cGAMP synthase cGAS and inhibit its enzymatic activity. cGAS binds to genomic DNA, thereby failing to activate expression of type I interferon (IFN) and maintaining the resting state of LT-HSCs. The study also found that cia-cGAS is a cGAS-mediated effective inhibitor of autoimmunity, providing new ideas and potential drug development targets for the effective prevention and treatment of autoimmune diseases and hematological malignancies. The research results were published in the internationally renowned academic journal Immunity (IF=22.845) in 2018 . (Chip experiment provided by Kang Cheng technical service) Long-term hematopoietic stem cells, as the highest potential stem cell line, have the highest ability to renew and differentiate, and provide continuous cell replenishment for short-term hematopoietic stem cells and pluripotent stem cells. Most of the time, LT-HSCs are in a dormant resting state, and their maintenance of dryness is affected by many factors such as transcription factors and bone marrow environment, such as TNF, CXCL4 and type I IFN. However, LT-HSCs are at rest. The fine-tuning mechanism between state and activation state has not been fully resolved. Firstly, the authors analyzed the circRNAs expression profiles of LT-HSC and MPPs isolated from mouse bone marrow by Arraystar Mouse CircRNA chip , and screened 156 differentially expressed circRNAs. The qPCR verification results were consistent with the chip. shRNA lentivirus transfection revealed that only the circRNA cia-cGAS transcribed by the D430042O09Rik gene can affect the subpopulation distribution of LT-HSCc. Subsequently, the authors found that cia-cGAS was highly expressed in the nucleus of LT-HSCs by qPCR, Northern blot, nuclear separation and in situ hybridization experiments. Result display Figure 1 Arraystar mouse circRNA chip screening and qPCR verification results and cia-cGAS expression analysis results, cia-cGAS high expression in LT-HSCs nuclei Figure 3. Results of the cia-cGAS mechanism. cia-cGAS binds to cGAS to inhibit its expression, thereby failing to activate the expression of type I IFN and maintaining the resting state of LT-HSCs. cia-cGAS is an effective inhibitor of cGAS-mediated autoimmune disease. Agent Significance In this study, a circus circus cia-cGAS with high expression of LT-HSCs was screened by Arraystar Mouse CircRNA Array . Functional mechanism experiments showed that cia-cGAS in LT-HSCs can inhibit the enzymatic activity of DNA-sensitive cGAMP synthase cGAS, which hinders cGAS. Combining genomic DNA, it is unable to activate the expression of type I IFN and maintain the resting state of LT-HSCs. At the same time, cia-cGAS is a cGAS-mediated effective inhibitor of autoimmunity, providing new ideas and potential drug development targets for the effective prevention and treatment of autoimmune diseases and hematological malignancies. Electrical Engineering,Winch Cable,Low Voltage Electrical Wire,High Voltage Electrical Wire Shandong Freedoms Technology Co.,Ltd , https://www.sdfreedomtech.com
Research Background
cGAS has been extensively studied as a cytoplasmic receptor. When DNA is bound, cGAS catalyzes the production of cGAMP, which binds to interferon-stimulating factors and induces activation of the cGAS-STING signaling pathway, thereby upregulating the expression of type I IFN and a series of immune responses. cGAS not only recognizes pathogenic DNA, but also recognizes the body's own DNA. In hematopoietic stem cells, how cGAS in the nucleus avoids binding to its own DNA and prevents autoimmune reactions and damage to the body is still rare.
CircRNAs are a large class of non-coding RNA molecules with closed loop structure, which play a regulatory role through various mechanisms. However, there are still few studies on the regulation of rk-HSCs resting state in circRNAs. The purpose of this study was to validate the circRNAs that regulate the resting state of LT-HSCc by circRNA microarray screening, and to study the mechanism of this regulation through a series of functional mechanisms to provide new ideas for the effective prevention and treatment of autoimmune diseases and hematological malignancies. Potential drug development targets.
Research ideas
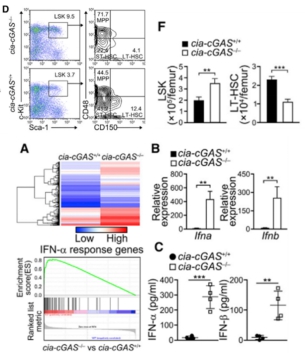
Next, the author studied the related functions of cia-cGAS. The authors specifically knocked out cia-cGAS by deleting the reverse complementary sequence downstream. FACS analysis showed that LSK cells increased significantly after knockout, LT-HSC decreased significantly, and the positive rate of BrdU staining of LT-HSCs after knockout was significantly higher than that of the control. Groups indicate that LT-HSCs are in an active proliferative phase after knockout. Pulse markers also showed a significant decrease in the resting state of LT-HSCs cells after knockout. The authors then found that type I IFN expression was significantly up-regulated by mRNA expression profiling in knockout mice, and qPCR and ELISA also confirmed this result. The above experiments indicate that cia-cGAS can regulate the resting state of LT-HSCs by type I IFN signaling.
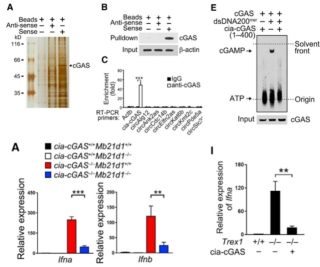
Finally, the authors explored the molecular mechanisms underlying the function of cia-cGAS. RNA pull down, RIP, immunofluorescence, EMSA and FISH experiments revealed that cia-cGAS can interact with cGAS proteins. Next, the authors demonstrated by enzyme activity assay, ChIP, HPLC, luciferase and mass spectrometry that cia-cGAS can block the binding of cGAS to LT-HSCs DNA, thereby inhibiting its enzymatic activity and not activating type I IFN expression. Then the authors used CRISPR/Cas9 mutant cGAS gene, qPCR, ELISA, BrdU labeling and other experiments showed that the expression of type I IFN was inhibited after mutation, and the number of LT-HSCs cells was normal and more maintained at rest. These indicate that the mechanism of cia-cGAS action is to inhibit the expression of type I IFN by binding to cGAS, and to maintain the resting state of LT-HSC. Animal experiments have shown that in cia-cGAS knockout mice, stimulation of poly(I:C) and HSV virus can lead to the production of a large number of type I interferons, thereby inducing autoimmune diseases, while cia-cGAS overexpression These can be eliminated, indicating that cia-cGAS is a potent inhibitor of cGAS-mediated autoimmune disease.
Technical route 
Original source
A Circular RNA Protects Dormant Hematopoietic Stem Cells from DNA Sensor cGAS-Mediated Exhaustion. Immunity, 2018.
[SCI 22 customer results] circRNA inhibits long-term hematopoietic stem cell depletion mediated by DNA receptor cGAS
Figure 2 cia-cGAS functional study results, cia-cGAS can regulate the resting state of LT-HSCs by type I IFN signaling
