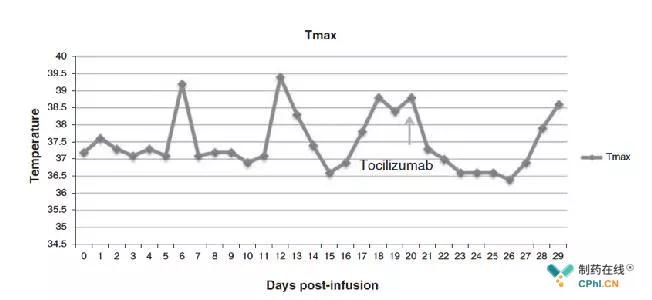
CAR-T 2.0: Amgen rheumatoid arthritis drug IL-1 antagonist Anakinra is expected to make CAR-T therapy safer! June 12, 2018 Source: CPhI Pharma Online Author: Knowing and Doing At this year's IBO International Conference, CAR-T 2.0 has once again entered the field of vision. Scholars hope that the next generation of CAR-T therapy will be more effective and safe. In terms of safety, studies have shown that in the mouse model, Amgen's rheumatoid arthritis drug IL-1 antagonist Anakinra can eliminate the occurrence of side effects of cytokine storms in CAR-T therapy, that is, IL-1 may be The initial cause of cytokine storms is expected to be validated in clinical trials as soon as possible and to make CAR-T therapy safer. Small seminar of BIO International Conference--CAR-T 2.0 CAR-T therapy is considered to be the most promising cell therapy for cancer. Since the treatment of blood tumors by Novartis and Gilead/Kite's CAR-T therapy in 2017, pharmaceutical companies and R&D institutions around the world have invested in CAR- The development of T therapy. According to statistics, there are currently 404 CAR-T projects in the world, and 152 (37%) in China, which has already entered the competition for CAR-T therapy. (Image from reference source 1) At the annual BIO International Conference, FierceBiotech will present a topic to invite participants to a small seminar. This year's theme is CAR-T 2.0. Following the approval of the first generation of CAR-T therapy, Kymriah and Yescarta, how to develop a wider application, better efficacy, and most importantly, safer CAR-T 2.0 therapy has become the goal of R&D institutions. It is well known that the current approved CAR-T therapy is only suitable for hematological tumors and its difficulty in the application of solid tumors. The scholars involved in the discussion are full of confidence in the treatment of solid tumors by CAR-T therapy. A large amount of evidence has proved that It has an effect in solid tumors. If the difficulties of the target and tumor microenvironment are overcome, it is bound to make CAR-T therapy take a big step forward in the application of solid tumors. In order to make CAR-T therapy safer and more effective, combination therapy has been mentioned many times, or with "old drugs" or with "new drugs", which requires more clinical trials to confirm which combination therapy is more rational. . Some scholars have mentioned that well-trained physicians may be more effective than drugs in controlling the side effects of CAR-T therapy. The key is to find a balance between clinical treatment design and CAR-T therapy. CAR-T therapy has considerable prospects in the treatment of tumors, especially hematological tumors, but its powerful tumor killing ability is a side effect that cannot be ignored - cytokine release syndrome. CAR-T therapy side effects CRS and existing coping treatment CAR-T cells scavenge large amounts of tumor cells in a short period of time, which will produce a "tumor effect", causing local inflammatory reactions and releasing a large number of cytokines (IL-6, IFN-, Fracktalkine, GM-CSF, IL-5). Etc.) causes side effects such as cytokine release syndrome (CRS). CRS can cause patients with high fever, rash and abnormal nervous system symptoms. Although clinical doctors can slow down the return of CAR-T cells to reduce the side effects of CRS, there are certain limitations in treatment. In September 2017, the FDA approved Genentech's IL-6 antagonist Actemra (Tocilizumab, originally used to treat rheumatoid arthritis (RA)) as adjunctive therapy for patients receiving CAR-T therapy to suppress its side effects CRS. However, Tocilizumab is not effective for every patient with side effects of CRS. As shown in the figure below, it has been reported that when the patient receives Tocilizumab treatment, the patient's temperature returns to normal after a period of time, but there is still a high fever. In addition, because Tocilizumab cannot break through the blood-brain barrier, it has nothing to do with neurotoxic side effects. Body temperature curve of patients from day 0-28 (chart from reference source 2) Amg en RA drug IL-1 antagonist Anakinra is expected to reduce the symptoms of CRS (Image from the network) Kineret (Anakinra), developed by Amgen for the first time in 2001 for the treatment of RA, is now likely to be used in the treatment of CAR-T side effects CRS, achieving "new use of old drugs." It is known that IL-6 is a cytokine that causes symptoms of CRS, but it is not the only one. Researchers from San Rafael Hospital have found that IL-1 is also an important cytokine. They found in the mouse model that Tocilizumab inhibits CRS by blocking IL-6, but when the IL-1 antagonist Anakinra is used, the side effects CRS disappear completely. Further studies have found that IL-1 has reached a high level before a few hours before the increase of IL-6. Part of the reason for the increase in IL-6 content is IL-1, which means that IL-1 may be The original reason for CRS. The researchers also found that if the Anakinra treatment was combined during the CAR-T cell reinfusion of the mouse model, the overall survival rate of the mice would increase significantly. In addition, the IL-6 antagonist Tocilizumab and the IL-1 antagonist Anakinra all have the effect of alleviating CRS, but have no effect on the neurotoxicity caused by CRS, Tocilizumab, which is a small molecule peptide that can penetrate the blood-brain barrier. It inhibits neurotoxicity, so Anakinra has an advantage over Tocilizumab. Two scholars from Memorial Sloan Kettering also demonstrated the important role of IL-1 in CRS. They found that IL-1, IL-6 and nitric oxide are involved in the development of CRS in mouse models and block IL-through by Anakinra. Treatment of 1 is more effective than blocking IL-6 to stop the occurrence of side effects. I hope that this research results will be verified in clinical trials faster, accelerate the development of CAR-T 2.0, and benefit more patients! Reference source: 1. FierceBiotech BIO breakfast-CAR-T 2.0: Biomedical science at the cutting edge; 2. Possible Compartmental Cytokine Release Syndrome in a Patient With Recurrent Ovarian Cancer After Treatment With Mesothelin-targeted CAR-T Cells; 3. Could an old Amgen-developed RA drug make CAR-T treatments safer?. Disposable Gel,Moisturizing Hand Sanitizer,Hand Sanitizer,Hand Sanitizer Dispenser Wuxi Keni Daily Cosmetics Co.,Ltd , https://www.kenicosmetics.com

CAR-T 2.0: Amgen rheumatoid arthritis drug IL-1 antagonist Anakinra is expected to make CAR-T therapy safer!
