Gaffer tape introduction
1. Material
Our gaffer tape is based on premium grade cloth fabric, combined with matt polyethylene, coated with synthetic rubber adhesive or natural rubber adhesive.
2. Features
Our Gaffer tape has following features:
a. Strong adhesion, we coated with high quality imported glue.
b. Heavy duty, it is made from strong material, thickness reaches 250micron, 270micron and 300micron.
c. Matt surface, non reflective.
d. No residue when removal.
e. Different colors for choosing. We have black, orange, pink, apple green, other colors also can be customized.
Gaffer Tape, Cloth Gaffer tape, Gaffers Tape, Gaff Tape, Black Gaffer tape, Gaffer duct tape Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytape.com
Profit margin
Recently, with the successive announcements of listed companies' 2011 financial statements, investors are happy and worried. Unlike the growing A shares, the financial reports of many domestic pharmaceutical companies are very bleak. Some companies’ profits have shrunk dramatically in 2011, and many have even dropped by 80% to 90%. Among them, the profits of mainstream anti-infective chemical companies in China showed a collective decline.
Similarly, several pharmaceutical companies with anti-infective medicines listed on the GEM are also the leading companies in the pharmaceutical sector.
Lukang Company believes that the reasons for the change in performance are the impact of the “limited resistance†policy such as the classification management of antimicrobial drugs, the decrease in the price of antibiotics in the domestic market, and the significant decline in prices of the company’s main raw material drug 7-ACA and related products. Second, with the national tender for essential drugs being implemented in various regions, the excessive competition in the price of some antibiotic products has caused huge negative effects on the market. In the second half of 2011, especially since the fourth quarter, the prices of the company's formulating products continued to decline. Third, due to high inflation during the reporting period, the prices of raw materials and energy needed for production of the company rose rapidly, and production and operating costs continued to rise. Fourth, the market competition has become increasingly fierce and product inventories have increased.
Most of the other companies interviewed by the reporter hold similar views: raw materials, power costs, and production and operating costs continue to rise. At the same time, since the second half of last year, various localities have gradually begun to increase the implementation of bid-in-aid bidding, and the tender price is generally low. The “limit-resistance order†and other policies have led to the shrinking of market capacity, the gross profit of the domestic antibiotic companies' formulation products has been greatly reduced, and the prices of bulk medicines have fallen sharply. .
In fact, in 2011, some traditional bulk varieties of anti-infective APIs were also not optimistic about exports. The data from the China Chamber of Commerce for Import and Export of Medicines and Health Products shows that in 2011, the export volume of raw materials in China was 5,883,200 tons, an increase of 27.8% year-on-year, but the export value of some bulk varieties declined. For example, the export amount of cephalosporin chemical raw materials decreased by 11.1% year-on-year, that of sulfonamides decreased by 79.64%, and that of penicillins rose slightly by 2.14%. "The market maintenance and growth of many products are realized at the expense of prices." The relevant members of the Medicare Chamber of Commerce said.
Outstanding advantages
It is the guiding direction of the current national industrial policy to optimize and upgrade the industrial advantages that we currently have in order to make it more internationally competitive. It is also the only way for domestic enterprises to enhance their competitiveness.
National People's Congress Xu Xuanren said: "China's innovation capability and foreign countries have a big gap, mainly Western medicine. In the Western drug R & D process, companies have grasped very tight, but to catch up with the level of innovative drugs in Europe and the United States, but also take time. â€
So, how can companies achieve transformation and upgrading? How can we get rid of the nightmare of overcapacity and the quagmire of low-price competition? How to shorten the time of catching up with the international advanced level?
ShiPai Group has a domestic class 1.1 new drug - Nbipu. This spent more than 3 billion R & D funds, tossing 12 years of innovative drugs before and after, in 2011 finally began to heavy volume.
“Enpreop sold 5 billion yuan in 2011.†According to relevant person in charge of the Shijiazhuang Group, the proportion of raw material drug revenue in the main business of the CSPC Group is declining year by year, and the innovative drug is becoming a new profit growth point of the Group. .
According to reports, Shiyao Group is currently researching 25 new drug varieties, some of which have entered different clinical stages. "In the next few years, after these new drugs are gradually put on the market, the company's performance will be blown out." The responsible person said.
The path taken by Ngpap made Cai Dongchen, chairman of the Shijiazhuang Group, feel deeply that the sound development of the pharmaceutical industry requires the government to create a favorable policy environment. Therefore, as the deputy to the National People's Congress, he submitted a proposal this year: He urged relevant government agencies to issue relevant policies as soon as possible to establish a favorable policy environment for innovative drugs and listed drugs to improve quality, and encourage pharmaceutical production R&D companies to increase the research, development, and quality of innovative drugs. Increase investment to improve the core competitiveness of China's pharmaceutical industry.
"Encouraging more companies to improve their quality management, improve the overall competitiveness of the pharmaceutical industry, and ultimately benefit the people is the common responsibility of the government and the enterprise," said Cai Dongchen.
In fact, we also see that North China Pharmaceuticals, Northeast Pharmaceuticals and other domestic chemical raw material drug companies are undergoing different transformation and upgrading process.
According to data from the Medical Insurance Chamber of Commerce, in 2011, exports of pharmaceutical preparations in China grew rapidly, and their growth rate was nearly 10 percentage points higher than that of raw material drugs. At the same time, rising export prices have boosted the export value. The penicillins, cephalosporins and other anti-infective drugs are still the main products of Chinese Western medicine exports, the cumulative export value of 558 million US dollars, accounting for 25.65% of China's Western medicine exports.
In this year's government work report, there is another paragraph that is quite encouraging to the industry: “Deepen the reform of science and technology system, promote enterprises to become the main body of technological innovation, and promote close integration of technology and economy. Support enterprises to strengthen the construction of R&D centers and undertake major national and regional scientific and technological projects. . "(author: Li Yao) 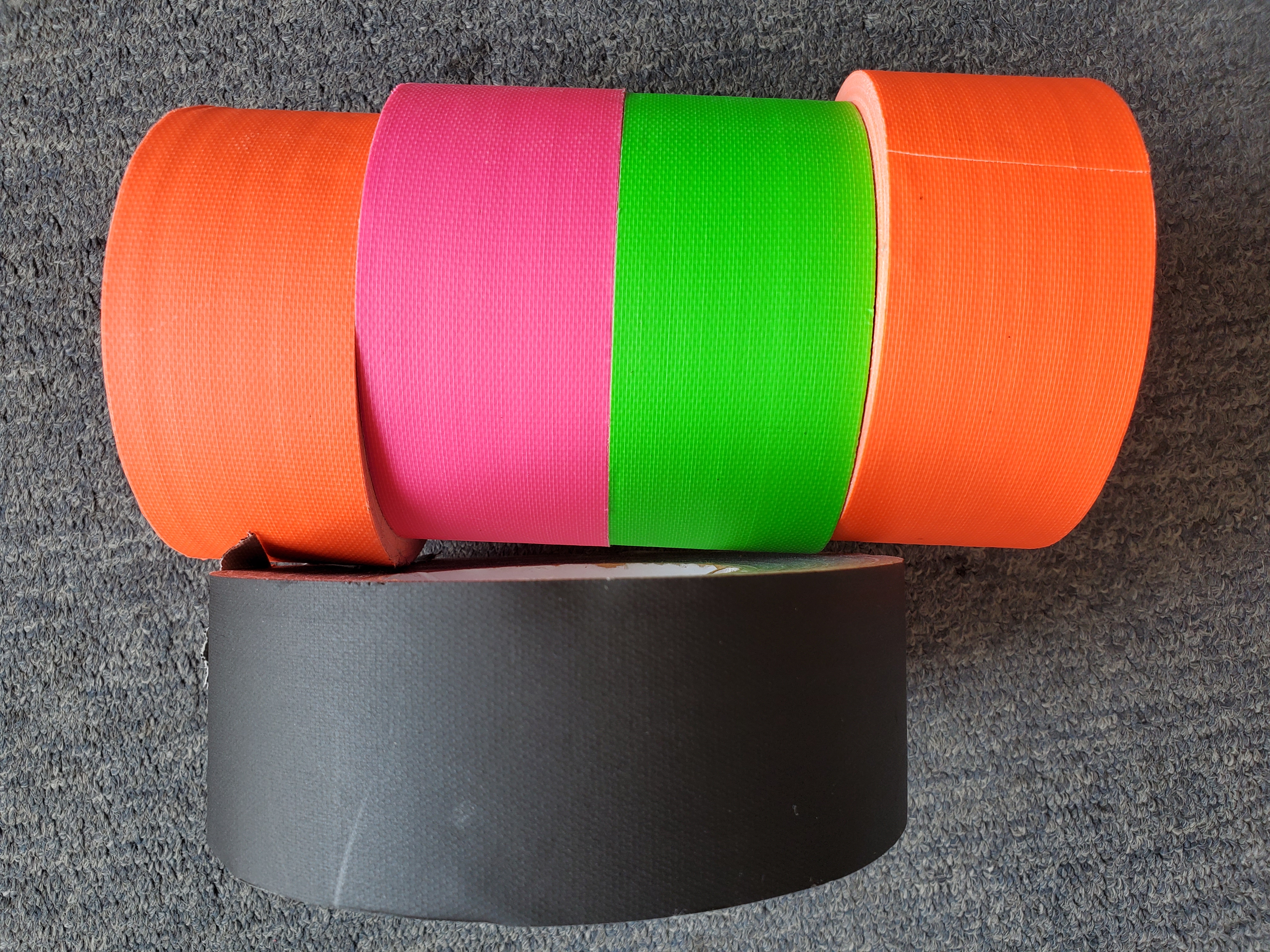
Pharmaceutical companies observe: Lost profits
The government work report of the “two sessions†this year pointed out: “Improve the optimization and upgrading of industrial structure. Promote the healthy development of strategic emerging industries.†At the same time, the report also pointed out that the development of high-end equipment manufacturing, energy conservation and environmental protection, biomedicine, new energy vehicles, and new Materials and other industries. We will increase the scale of special funds for technological transformation and promote the upgrading and upgrading of traditional industries. In this regard, the pharmaceutical industry has also taken action.
