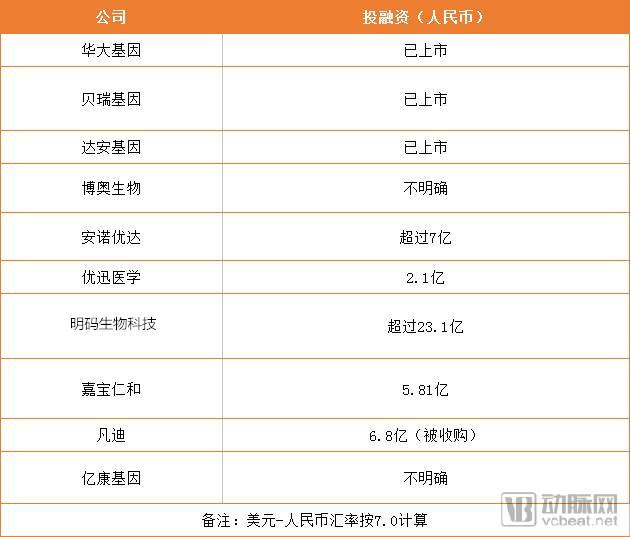
According to estimates by the World Health Organization, the incidence of birth defects in low-income countries is 6.42%, that of middle-income countries is 5.57%, and that of high-income countries is 4.72%. The incidence of birth defects in China is close to the average level of middle-income countries in the world, but due to the large population base, the total number of new birth defects per year is huge. A few days ago, the 2018 Pediatrics Annual Meeting was held as scheduled, and birth defects prevention and control were mentioned many times during the meeting. The prevention and control of birth defects in China can be divided into three levels of defense. The first level is genetic pre-pregnancy screening, the second level is prenatal series of serology, imaging, and genomics screening. The third level of defense is Newborn screening. Despite the three defenses, there are about 900,000 newborn birth defects in China each year, of which genetic birth defects account for about 30% of total birth defects. Moreover, in the neonatal stage, the phenotypic characteristics of many genetic diseases are not typical and may not be directly observed. Although ordinary children look healthy, they may also be potential patients with genetic diseases. The phenotype of this part of the genetic disease is gradually appearing in growth and development. Once the disease occurs, it will cause serious harm to the child's health and bring great suffering and burden to the family. 1. Fundamental: Birth defects have become the primary cause of infant death in China The incidence of birth defects in China is close to the average level of middle-income countries in the world, but due to the large population base, the total number of new birth defects per year is huge. According to the data of the 6th Birth Defect Prevention and Control Conference of the National Health and Welfare Department’s Leading Women’s and Children’s Department’s Report on the Progress of Prevention and Treatment of Birth Defects in China, the statistics on the national death monitoring system for children under 5 years old are about 1 in every 5 infant deaths. One died of birth defects, and three babies die every hour from birth defects. Birth defects have become the leading cause of death in infants in China. It was mentioned in the latest National Plan for Comprehensive Prevention of Birth Defects published in August 2018 that birth defects prevention and control will be integrated into all health policies. Adhere to the combination of prevention and treatment, and improve the full service of prevention, screening, diagnosis, treatment and rehabilitation. Specific objectives: By 2022, the awareness rate of birth defects prevention and control knowledge reached 80%, the pre-marital medical examination rate reached 65%, the pre-pregnancy eugenics health check rate reached 80%, and the prenatal screening rate reached 70%; neonatal genetic metabolic disease screening The investigation rate reached 98%, the hearing screening rate of newborns reached 90%, and the treatment rate of confirmed cases reached 80%. 2, gene technology to help birth defects prevention and control: not only NIPT Since genetic testing can be used for the detection of genetic diseases, it is important to prevent birth defects. The third-level prevention work of birth defects needs to focus on promoting the comprehensive prevention and treatment of birth defects and promoting the scientific application of genetic testing technology in the comprehensive prevention and treatment of birth defects. NIPT is a masterpiece of genetic technology in the prevention and control of birth defects. But in addition to secondary prevention, genetic technology companies are also expanding to primary and tertiary prevention. NIPT is the first commercial gene sequencing technology. Since its inception in 2008, it has been a Red Sea market. However, the application of genetic technology to Level 1 and Level 3 defenses does not involve a large number of companies. In contrast, this is still a blue ocean market. From the above table, we can see that in addition to NIPT, several industry giants have more or less pre-pregnancy and newborn screening stages. Berry's gene was first introduced in 2013 as a chromosomal aberration detection product, Conoan. In addition to its use in pregnancy testing, this product can also be used for pre-pregnancy screening in families with a family history of chromosomal genetic diseases. In August 2016, Berry's "preimplantation embryo chromosomal aneuploidy test kit" was approved by CFDA for innovative medical devices. The launch of this product marks Berry's gene coverage of prenatal, pre-implantation, pre-natal, and neonatal birth defects prevention services and product layout. In fact, as early as 2014, Berry Gene successfully developed a genome-wide detection technique for pre-implantation embryos at the single-cell level in conjunction with the General Hospital of the Chinese People's Liberation Army, and published the results in the Journal of Genetics and Genomics. In 2016, the comprehensive solution for Konoan's chromosomal diseases was comprehensively launched, including a series of complementary products from DNA extraction to report production. In addition to prenatal, it can also be used for abortion reasons, infertility causes and suspected chromosomal diseases. Detection of chromosomal diseases. In addition, there are screening products such as Beicong and Xixin (SMA, FXS screening). After launching NIFTY for 1 year, Huada Gene has launched a deafness genetic screening product. By collecting micro blood, it can detect the risk of congenital, late-onset and drug-sensitive deafness after 5-15 days of birth. In the following years, Anyangke, Anxinke, and thalassemia gene detection products were successively introduced, and Huada Gene gradually improved the product layout from pre-pregnancy to neonatal period. In 2009, Boao Bio has developed the first international genetic testing kit for hereditary deafness, which provides effective support for the prevention of hereditary deafness. As early as 2011, the company launched a genetic screening program for high-risk populations in Beijing. Subsequently, the company continued to follow up in Chengdu, Changzhi, Nantong, Zhengzhou, Changchun and other areas, and continued to extend to Shanghai, Fujian, Shandong, Yunnan, Jiangsu, Hunan, Jilin, Zhejiang, Guangdong, Anhui, Gansu, Guizhou, Xinjiang and other regions. . It is understood that the total number of people using deafness gene detection chip screening has exceeded 1 million. Annoyouda is a rising star in the field of NGS, and its research services are an important part of its business. In the product layout of prevention and control of birth defects, NIPT products have been produced. In recent years, the company has also introduced testing products covering pre-pregnancy and neonatal period, such as folic acid metabolism, deafness gene and thalassemia, and completed its research from upstream to downstream. + Multiple natures such as clinical, birth defects and tumors. U-Med is more focused on the prevention and control of clinical tumors and birth defects. Out of the high frequency of folate metabolism, thalassemia and deafness gene detection, they also launched a physical examination products during pregnancy, from pre-pregnancy to neonatal period, and even physical examination during pregnancy. Mingma Biotech's mission is to apply precision medicine big data to improve human health. Previously, Mingma Biotech's business in the field of gene sequencing has multiple data services, and the company cooperated with Huawei Cloud and Alibaba Cloud. And by the beginning of 2016, the company began to introduce substantial products, starting with the prevention and control of birth defects. In December 2016, Mingma Biotech officially released the pre-pregnancy carrier screening product - "Fu code". Fufa is a non-invasive pre-pregnancy genetic disease screening product launched by Mingmao Biotechnology, which can screen 135 kinds of serious recessive genetic diseases most common in Chinese. In the past two years, Mingma Biotech has once again joined the Shanghai Fudan Children's Hospital to release the "Xin code" for newborn genetic screening products. The two sides indicated that the product can achieve the coverage of hundreds of common genetic diseases of newborns, and it is expected to promote the development of precision medicine in China, especially the tertiary prevention of neonatal genetic diseases. 3. How do you think about capital? From the perspective of national strategy and market demand, these companies are stepping on the express technology of genetic technology to do things that conform to the development of the times. But as with all high-tech industries, from early technology to productization and marketization, this is inseparable from market support. So what does capital look like in this field? From the data point of view, there are three listed companies here, and Boao Bio is a Tsinghua company. Most of Huada’s income is actually derived from birth defects, and Berry’s early business is also prevention of birth defects. Only prenatal screening gave birth to two listed companies. Looking at non-listed companies, Annoyouda and Youxun Medical belong to the three-level defense system for the prevention and control of birth defects. The two companies have total financing of more than 700 million yuan and 200 million yuan respectively. Among them, the valuation of Annoyouda has been More than 4 billion yuan. In contrast, Mingma Biotech did not go to the pre-production market, focusing on pre-pregnancy and neonatal stages. They plan to use big data to help prevent birth defects. In the three years since its establishment, the company has also raised $330 million. Among them, in 2017 alone, the company completed two rounds of financing, with a total amount of financing of 315 million US dollars. The list of investment institutions includes capital bulls such as Sequoia Capital, Temasek and Yunfeng Fund. In addition, Vandy Bio was acquired by Sanpower Group for RMB 680 million, which set a record at that time. "NIPT products have made great contributions to the prevention and control of birth defects in China." Professor Zhou Wenhao, deputy dean of the Pediatric Hospital affiliated to Fudan University, said, "But from the current situation in China, the light is simple. Link prevention is not enough." 4, newborn screening, the last part of the birth defect remedy Primary prevention is a carrier screening from a genetic perspective, and secondary prevention is prenatal. Despite this, there are still about 900,000 newborns born with birth defects every year. There are also some three-level prevention methods in clinical practice, such as tandem mass spectrometry. "But the problem is at the genetic level, and it still needs technical means of genetic testing to see it clearly," he added. Professor Zhou Wenhao revealed that in developed countries, 40% of the causes of death among children under 5 years of age are genetic factors. The more developed the economy, the more obvious the weight of heredity in the cause of death. "Because other problems have been overcome, but genetic problems are still in front of them," he said. In August 2016, Fudan Children's Hospital and Mingma Biotech jointly established a joint laboratory. Two years later, the two sides jointly launched the "Xin code" of the newborn genetic screening product, hoping to add the last link to the prevention and control of birth defects. "We need a positioning system to find out the coordinates of the disease so that the doctor can find a solution," he said. 5. The future: the power of data But is it enough to have a product come out? Although the cost of gene sequencing has dropped to a low enough level, it is not uncommon to look at the market for cancer detection products. "As a doctor, we hope that the product can be clinically helpful, fast, accurate and cheap." Asking what kind of product is clinically necessary, Professor Zhou Wenhao answered. He believes that testing products must be fast enough to meet the requirements of clinical decision-making; at the same time, this product should be accurate enough, and the results should not be ambiguous; it should not be overlooked that a product has a wide coverage and the price is low enough. "We want to be able to use the same products as blood routines, let patients spend a small amount of money and get things done," he continued. Indeed, technically, the application of genetic testing in clinical testing has matured. But to make the product universal in the clinic, price, accuracy, operation, and scale determine whether the product and the company can stand out. How can it be achieved? We also reviewed the trends of several leading companies in recent years and found that big data may be a path. Yuan Jianzhong, general manager of Mingma Biotechnology, told the arterial network that the first generation of Xinxin's prototype needs to detect more than 3,000 sites. After a large sample of clinical validation, they reduced the detection site to 300, and can cover more than 100 diseases. “To avoid unnecessary testing, the cost will naturally come down,†he said. In addition to Mingma Biotech, other companies are also practicing the road to big data. Since 2016, Berry Gene has launched the Shenzhou Data Cloud Project to complete the construction of the world's first Chinese population genome database, filling the gap in the international genetic database that lacks the unique genomic data information of the Chinese population. In 2017, the Berry Gene after the listing was invested in building a big data industrial park in Fujian. The industrial park is based on the big data of the Chinese population's disease-causing gene information database. Through cloud computing, gene sequencing, gene editing, artificial intelligence and other technical means, it builds an ecosystem covering the four major sectors of production, learning, research and capital. system. “This is a market that we think is emerging.†This is Zhou Daxing’s evaluation of the big data industry in an interview. He believes that the current gene big data is still in a fragmented state and has not yet formed a link in the industrial chain. However, he stressed that from the current phenomenon, the improvement of data processing and storage capacity will become an essential foundation for the next competition in the industry. For Mingji Biotech, in addition to the iterative upgrade of test products, another important use of gene big data is in the development of new drugs. After finding the cause, not all diseases can find a solution. For unmet needs, these accumulated data can provide important guidance for the development of new drugs for pharmaceutical companies. Mingma Biotech itself has a lot of data solutions. Yuan Jianzhong told the arterial network that in the course of clinical trials of drugs, the clinical effects are often not as expected. This is actually due to the screening of patient samples. Only the apparent morphology was considered in the clinical enrollment, and the genetic differences of patients without the same disease were not considered. The addition of gene big data can reclassify patients from the genetic layer, combined with proteomics and clinical phenotypes, can improve the effectiveness of new drug development. Similarly, the development of drug targets relies heavily on molecular diagnostic techniques, and a large number of clinical samples can provide direction for new drug development in pharmaceutical companies. We often say that children are the flowers of the motherland, but some of these flowers are accompanied by painful growth. "Pregnant and eugenics" is China's basic national policy, and in order to improve the quality of the population, both the state and the enterprise have done a lot of efforts. But as Professor Zhou Wenhao said, after other problems are solved, the problem of genetic factors is highlighted. In the next prevention and control of birth defects, the resolution of genetic defects may become the primary task. External Aluminum Foil Reflective Series External Aluminum Foil Reflective Series,Sunshade Net with Grommets,Wind Resistant Outdoor Shade Net,Insulation Sun Shade Curtain Changzhou Green Nets Co.,Ltd. , https://www.gnshadenet.com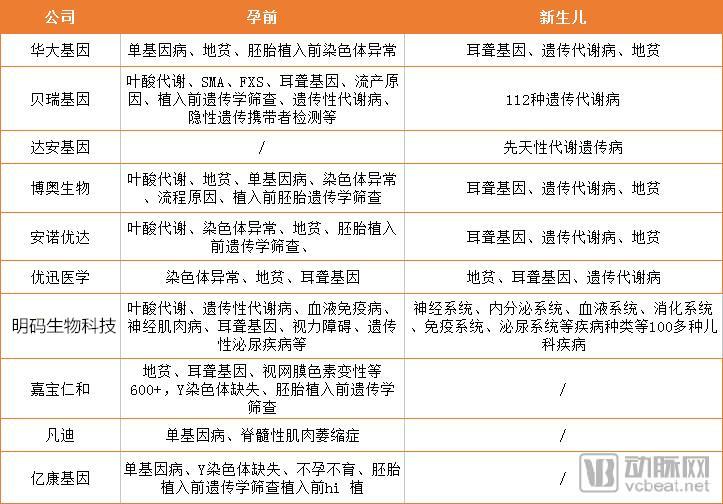
More than NIPT: The birth defects prevention and control market is moving from the middle of the Red Sea to the blue ocean at both ends
