Business Club April 18 News For many large pharmaceutical companies, 2010-2011 is a difficult time. Many "bomb bomb" drugs, such as Pfizer's Lipitor and Lilly's Zabula, will expire. Loss of market exclusive rights. The analysis predicts that 2011 will be a buffer year. Sales of genuine generic drugs will occur in 2012. It is expected that the patented drugs will lose about 33.2 billion U.S. dollars in the United States due to generic drugs, which is a 1X increase from the 15.8 billion U.S. dollars this year. Earlier this year, Polivi, which was jointly developed by Bristol-Myers Squibb and Sanofi-Aventis, received protection for a six-month extension of the patent period in pediatric applications, which was extended from December of this year to May next year. Analysts believe that Polly Wei's generic invasion will begin in mid-2012. Although GlaxoSmithKline’s Schlieddie’s patent expired this year, it is expected that the FDA will not approve Seridit’s generic drugs in the short term due to the difficulty of imitation. It is reported that Teva and Sandoz, the world's major generic drug makers, have given up the imitation of this drug, so that by 2016, there will be almost no Seridean generic drugs listed in the United States. Therefore, GSK is short-term. There is no need to worry about the risk of sales falling due to the expiration of the patent. As a result of the patent litigation, Takeda's Diabetes “bombshell†product, the extension of the patent period of Accor, was extended for 19 months. From 2011 to 2012, it was undoubtedly a major patent victory for Takeda. Lipitor’s patent expired in December of this year, and the Indian generic company Lan Boxi as the first patent challenger won 180 days of exclusive marketing rights in the United States. Generic drugs led to a dramatic drop in the sales of Lipitor in the United States beginning in 2012. With the launch of a variety of generic drugs, the drug sales volume will drop to less than US$150 million by 2013. Five "bombshell" drugs will expire this year, and 10 drugs with annual sales of about 1 billion U.S. dollars will expire in 2012 (see table). For Novartis, the expiration of the patent resulted in a decline in the sales volume of the antihypertensive drug in the United States, which did not reach the top five. In 2010, the sales of the article reached US$2.5 billion. AstraZeneca, which has FDA-approved Scarletzer XR for adjuvant treatment of major depressive disorders in adults, offsets the fall in Serocom’s sales, while the company has also developed sustained-release tablets that can be sold globally and patented until 2017. Anjin's "bombshell" class biologics, arthritis treatment drug Enli, is expected not to be affected by generic drugs in the next few years. This is because the American biosimilar drug policy is not yet clear and will allow Amian to sit back and relax. In addition, it is worth mentioning that in the next few years, some “bombshell†biologics will also expire their patents. For example, Roche’s Meritus’ patent expires in 2014, and Sanofi’s Aventis’s arrival (Lantus). At the expiration of the 2015 patent, Abbott’s Humira expired in 2016. Due to policy reasons, it is still uncertain that biosimilars of such products will enter the market. Patent litigation may play a key role in the next few years and it is full of variables. For example, Momenta Pharmaceuticals successfully shut down the Sanofi-Aventis Lovenox patent period by 2 years in advance in 2010. Teva's arthritis drug Copaxone is also expected to defend the patent period until 2014. Disposable Plastic Aprons,Plastic Aprons,Disposable Aprons,Disposable Polythene Aprons Surgimed Medical Supplies Co.,Ltd , https://www.surgimedcn.com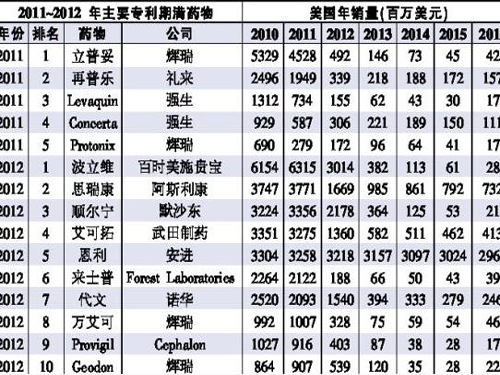
"Heavy **" drug patent expires 2012 generic invasion
