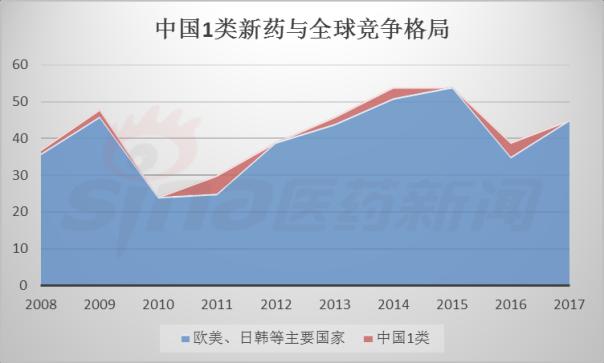
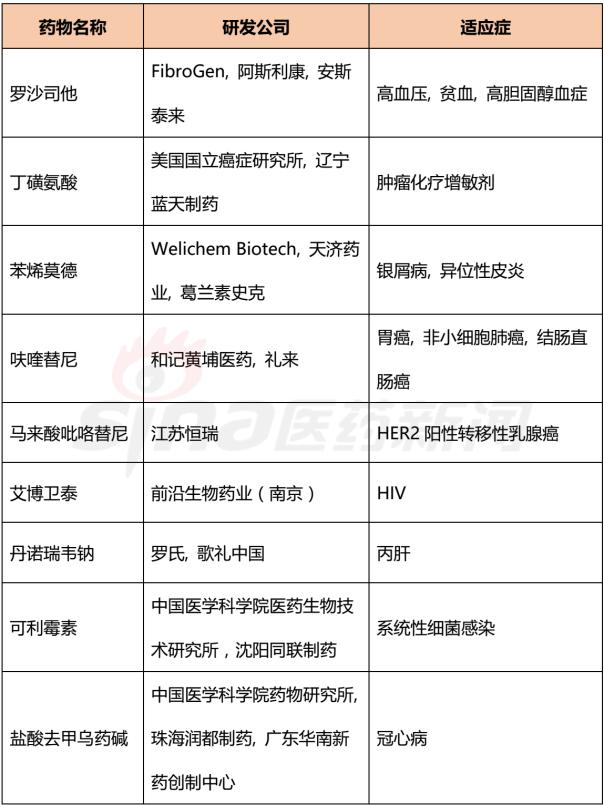
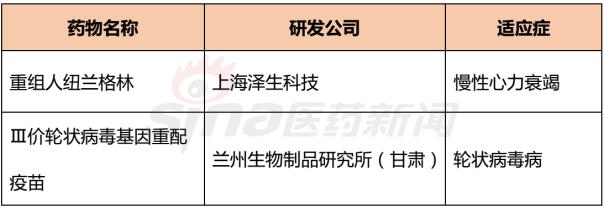
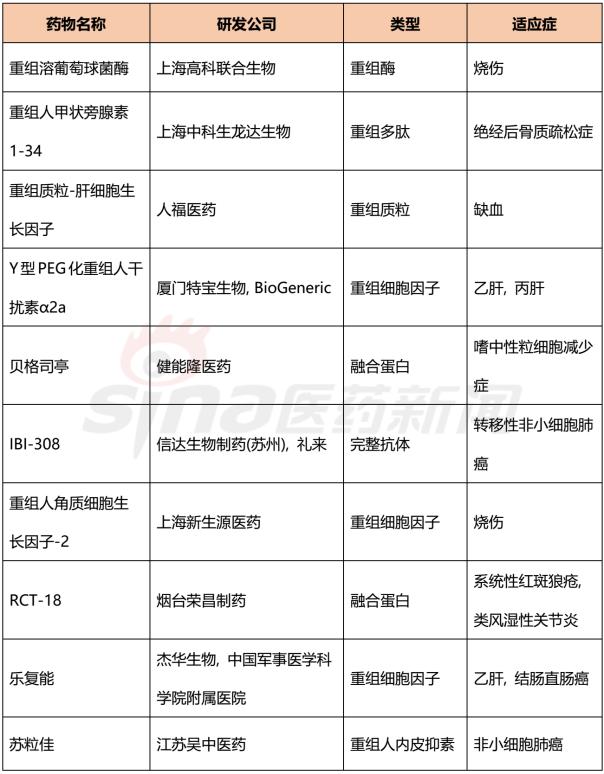
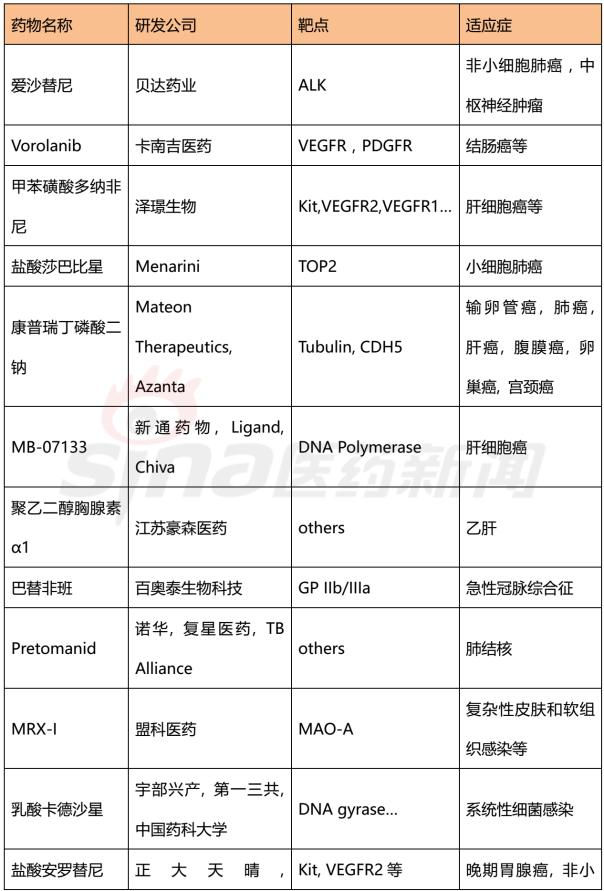
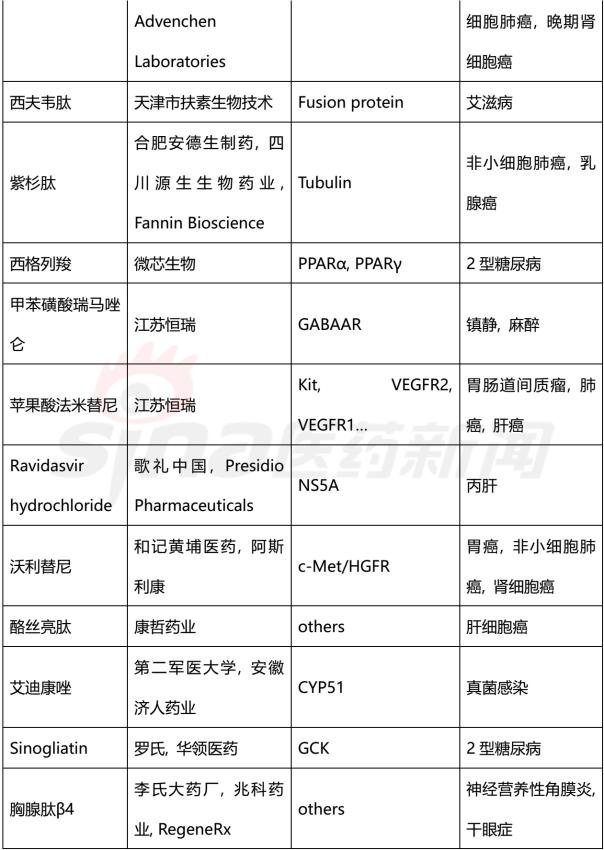
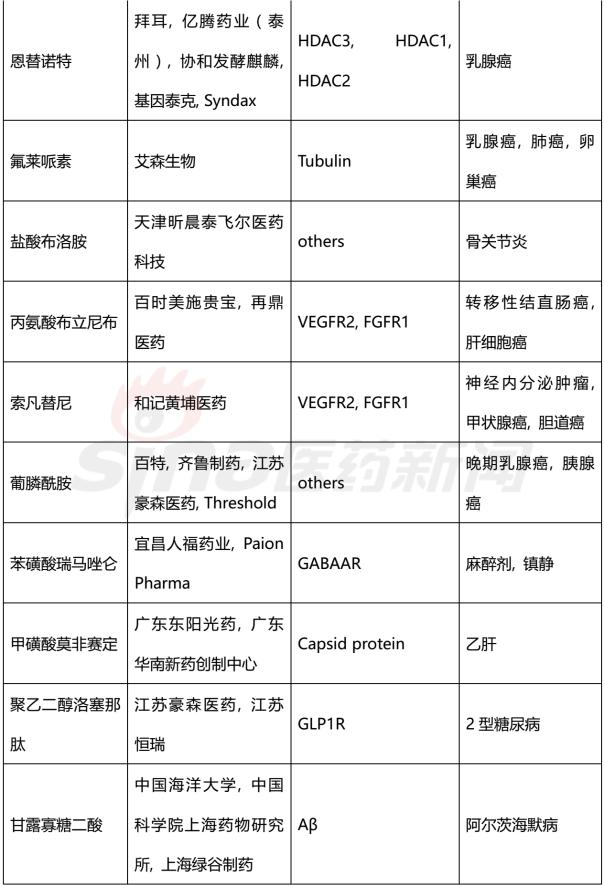
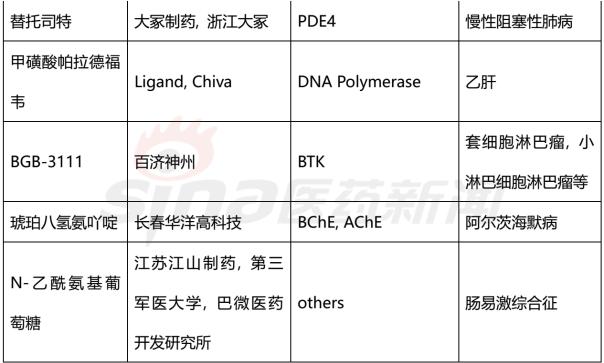
Progress in Clinical Research of Class 1 New Drugs in China in 2017 November 23, 2017 Source: Sina medicine Author: Dina Since March 4, 2016, after the CFDA issued a work plan on the reform of the chemical registration classification, China's Category 1 has been redefined as an innovative drug that is not listed at home or abroad. It refers to a new well-defined, pharmacologically active chemical. Drugs with clinical value. On the basis of the material foundation originality and novelty, while emphasizing clinical efficacy, it also began to connect with the world. According to the drug data index, from 2001 to 2016, CFDA approved 13 listed 1 chemical drugs and 16 biological drugs. The global (Europe, Europe, Japan, South Korea and other major countries) from 2008 to 2017 approved a total of 256 listed chemical drugs, biopharmaceutical 146 (including biosimilar drugs), the gap is obvious. 1. China's Class 1 in clinical application As of November 15, 2017, there are still no new class 1 drugs listed, but there are 9 types of class 1 drugs in the new drug listing application (NDA) stage. The indications include cardiovascular, tumor, HIV, infection and many other hot spots. field. Table 1 Chinese Class 1 New Drugs in 2017 NDA Application >>>>Rossastat Rosapstat is a hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor that inhibits the ubiquitination of hypoxia inducible factor (HIF) and helps the body produce more erythrocyte. Roscha was originally developed by FibroGen (Zibo Jin) and in 2006 authorized Anstay to develop rights in Asia, Europe and South Africa. In 2013, it authorized the development rights of AstraZeneca to the United States, China and major regions other than Japan and Europe. In April 2014, the compound was reported by the Zibo Jinji Pharmaceutical Technology Co., Ltd. in China, and the clinical approval was obtained in August 2015. >>>>Busulline In China, sulphonine is jointly developed by Liaoning Blue Sky Pharmaceutical, Fudan University and Shanghai Zhongmin New Technology. It is a tumor chemotherapy sensitizer. The compound obtained the first phase clinical certificate of new drug in 1998, the second and third phase clinical drug certificate in 1999, the second phase clinical trial in 2004, the third phase clinical trial in 2007, and the production in September 2011. Complement some clinical trials. In March 2014, he obtained a Class 1.1 clinical approval for Chinese chemical medicine. Butylsulfonate was developed in the United States by the National Cancer Institute for the treatment of neuroblastoma and is currently in clinical phase. >>>>Benzene Benzenemod filed a new drug listing application (chemical class 1) in December 2016 for the treatment of dermatitis and psoriasis. The compound was originally developed by Welichem Biotech and later licensed to Steve (Grace Sussex) and Tianji Pharmaceutical. >>>>Fuqualinib Furofinib was originally developed by Hutchison Whampoa and was authorized by the company to develop in 2013. For the treatment of gastric cancer, advanced or metastatic colorectal cancer and non-small cell lung cancer. In June 2017, the NDA application for furazolinib in the treatment of advanced colorectal cancer was officially accepted by the China Food and Drug Administration (CFDA). Furazolinib is a highly selective oral inhibitor of vascular endothelial growth factor receptor (VEGFR), which has the potential to be the best-in-class VEGFR inhibitor in the world for the treatment of multiple solid tumors. Based on previous preclinical and clinical studies, the kinase selectivity of furitinib has been shown to reduce off-target toxicity. Under these conditions, drug exposure can completely inhibit VEGFR, and the potential can be used in combination with other targeted therapies and chemotherapy in a larger patient population receiving early treatment (VEGFR is a receptor that contributes to neovascularization around the tumor) Body tyrosine kinase, which contributes to tumor growth). Efficient, low toxicity, suitable for combination therapy is an excellent property of furazolinib distinguished from other approved small molecule VEGFR inhibitors. Hutchison Whampoa is currently working with Lilly to conduct research on the treatment of colorectal cancer, non-small cell lung cancer and gastric cancer with furazotinib in China. In addition, a factory was set up in Suzhou and equipped with a complete set of production facilities for the production of furazolinib capsules. >>>>Pirrotinib maleate Pyrrolidine maleate was developed by Jiangsu Hengrui and submitted for marketing in China in August 2017 for the treatment of HER2-positive metastatic breast cancer. In May 2011, Jiangsu Hengrui Medicine and Shanghai Hengrui Medicine jointly submitted a clinical trial application (Chemical Drugs 1.1) to CFDA, and in April 2012, obtained a Chinese clinical medicine 1.1 clinical approval. >>>> Aibo Weitai Abbott is a human immunodeficiency virus (HIV-1) fusion inhibitor that is effective against the predominantly HIV-1 and drug-resistant viruses and treats HIV-1 infected people who have received antiviral therapy. In July 2016, leading-edge organisms submitted an NDA application (chemical class 1) to CFDA. >>>>Danovir sodium Danolitis sodium was filed in China in December 2016 for an NDA application, HCV protein inhibitor, in combination with peginterferon, ribavirin and ritonavir for hepatitis C infection. The compound was originally developed by InterMune (R&D code ITMN-191, ITMN-B) and was licensed to Roche in 2006 (R&D code R-7227, RG-7227, RO-5190591), which was terminated in 2010, Roche acquired In the research and development of drugs, InterMune was acquired by Roche in 2014. In 2013, Roche authorized the research and development rights of China and Taiwan to the songs (R&D codes ASC-08, AR-00334191, AR-334191). >>>>Cholicimycin Colimycin is a macrolide antibiotic that treats bacterial infections. Colimycin has been researched and developed by the Institute of Pharmaceutical Biotechnology of the Chinese Academy of Medical Sciences and has been transferred to Shenyang Tonglian Pharmaceutical Group. In September 2010, the compound was jointly declared by the Institute of Medical Biotechnology of the Chinese Academy of Medical Sciences, Shenyang Tonglian and Beijing Shouke Group. >>>>Naephrine hydrochloride Norfloxacin hydrochloride is in the declared production period, beta adrenergic receptor agonist, used to treat coronary heart disease. In August 2012, Zhuhai Rundu Pharmaceutical, Guangdong Huanan New Drug Creation Center and the Institute of Materia Medica of the Chinese Academy of Medical Sciences jointly announced the production review of the Chinese medicine 1.1 category. There are 2 types of biopharmaceuticals in the Biological Product Licensing Application (BLA), which are expected to be listed next year. Table 2 Biological drugs in the 2017 BLA application >>>>Reorganized people Newland Green Recombinant Human New Zealand Green is a recombinant protein drug derived from the naturally occurring active polypeptide fragment of Neurogulin-1 in humans, which mediates ErbB2/ by binding to the ErbB4 receptor of the epidermal growth factor receptor family member on the surface of cardiomyocytes. The formation of ErbB4 heterodimers activates a series of downstream signaling pathways, which play a role in repairing myocardial cell structure and improving cardiac function. This medicine can be used to treat moderate to severe chronic heart failure (CHF). The reorganized Newland Green was originally developed by Shanghai Zesheng Technology Development Co., Ltd. In 2013, SciClone obtained the commercialization authorization of the drug in China, Hong Kong and Macau. In April 2012, Shanghai Zesheng Technology submitted to CFDA the production application of recombinant New Zealand Green (1 category for therapeutic biological products). >>>>III price rotavirus gene reassortment vaccine The III-valent rotavirus gene reassortment vaccine developed by the Lanzhou Institute of Biological Products is used to prevent rotavirus infection. In November 2016, the Lanzhou Institute of Biological Products submitted the application for the production of this vaccine to CFDA (Class 1 for the prevention of biological products). Second, the Chinese class 1 in the clinical research stage As of November 15, 2017, there are 506 new classes of drugs in the clinical research phase, including 52 in clinical phase III, 75 in clinical phase II, and 300 in clinical phase, and in clinical applications. There are 79. The following is an enumeration of chemical and biological drugs in the third phase of clinical practice. Table 3 10 varieties of biopharmaceuticals in phase III clinical trials Table 4 38 varieties of chemicals in phase III clinical trials (This article data source: Drugs Data Pro V2.0)
We're Professional Supplier Extract Powder manufacturers and suppliers in China specialized in providing high-quality products at low price. We warmly welcome you to buy or wholesale bulk Supplier Extract Powder for sale here from our factory. For a free sample, contact us now.
Supplier Extract Powder,Supplier Extract ,Supplier Powder Manufacturer in China Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.bio-pharmacies.com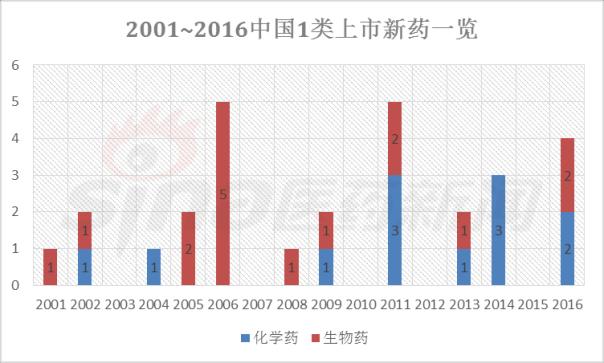
Progress in Clinical Research of Class 1 New Drugs in China in 2017
