Hcg Pregnancy Midstream,Midstream Pregnancy Test,Hcg Pregnancy Rapid Test Midstream,Early Midstream Pregnancy Test Weihai Kangzhou Biotechnology Engineering Co.,Ltd , https://www.weihaikangzhou.com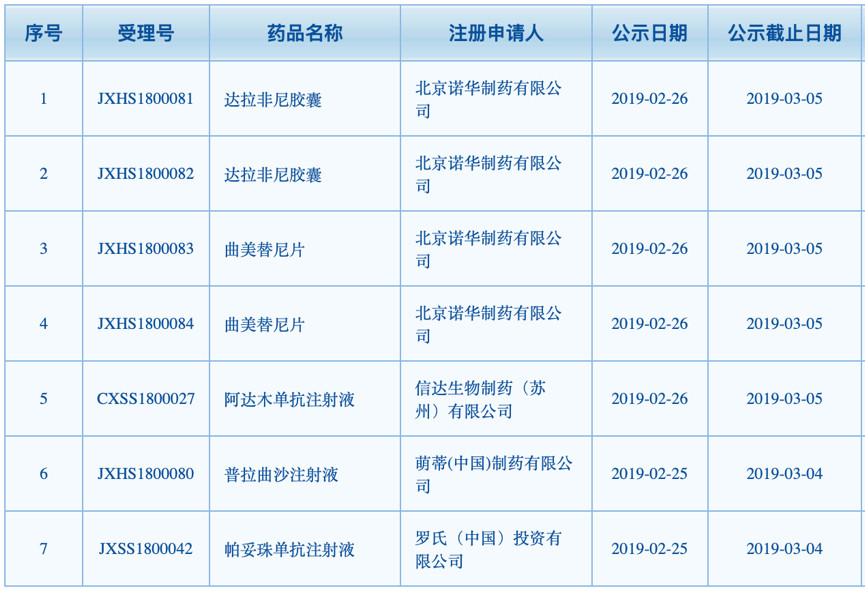
A batch of new drugs or listed in advance! Zhengda Tianqing, Hengrui, Haosen...
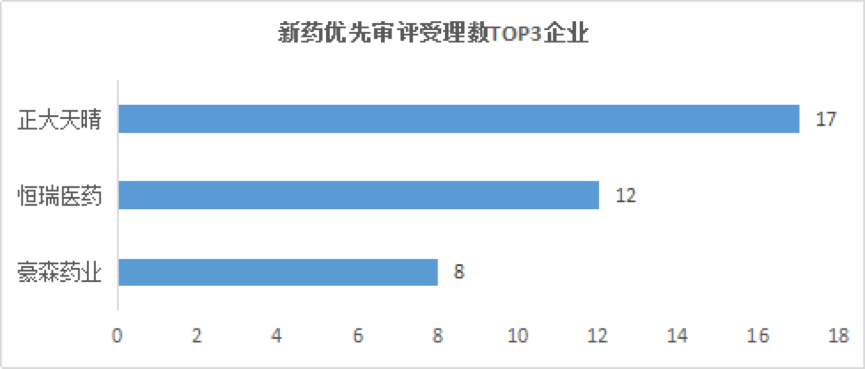
Medical Network February 28th Among the enterprises that applied for new drugs, Zhengda Tianqing had the largest number of acceptances, 17; Hengrui Medicine and Haosen Pharmaceuticals followed, 12 and 8 respectively.
â– A batch of new drugs, or listed in advance
On February 26, the National Drug Evaluation Center was proposed to be included in the priority review and approval column, adding 7 drugs, a total of 5 varieties, including Beijing Novartis's darafini capsules and trimetini tablets, Cinda's Ada. Muzumab injection, Mengti Pharmaceutical's Prazera injection and Roche's Pertuzumab injection.
â–7 billion market, two new drugs listed in advance? !
According to medical geography, both darafini and trimetinib are developed by GSK and are now owned by Novartis. The reason is that the GSK and Novartis business exchanges in 2014-2015: At that time, Novartis acquired GSK's oncology business for $14.5 billion, and Novartis will also add $71 million to the vaccine business in addition to the flu vaccine. The price is transferred to GSK.
Data show that darafini, the FDA approved in 2013 for the treatment of metastatic melanoma and melanoma patients who cannot be treated surgically. It is the third drug for the treatment of metastatic melanoma approved after vemurafenib and iprezumab, and trimetinib is the first FDA approved for advanced melanoma patients carrying the BRAF V600E/K mutation. A kinase allosteric inhibitor.
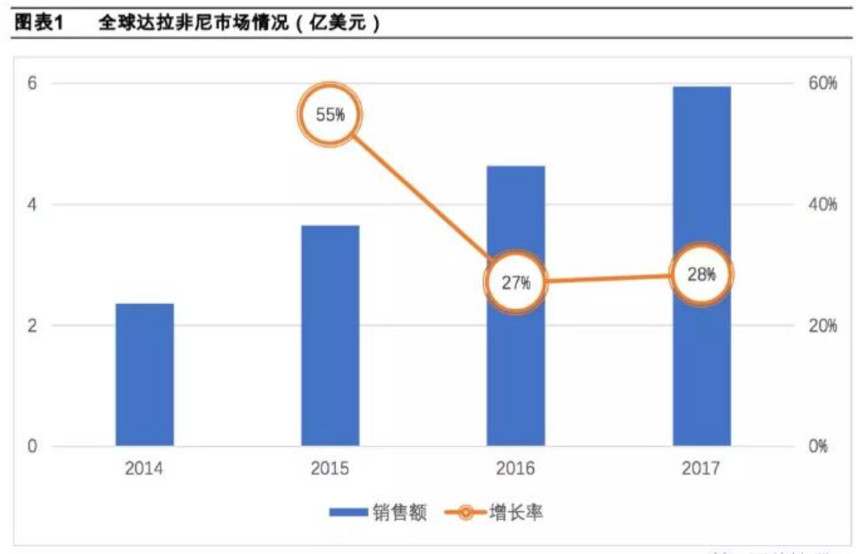
According to the Comprehensive Drug Database (PDB), Dalafini has maintained steady growth in 2014-2017, with global sales of nearly 600 million US dollars in 2017. After a rapid growth of 55% in 2015, it gradually became 2016-2017. stable.
(Source: Medical Geography)
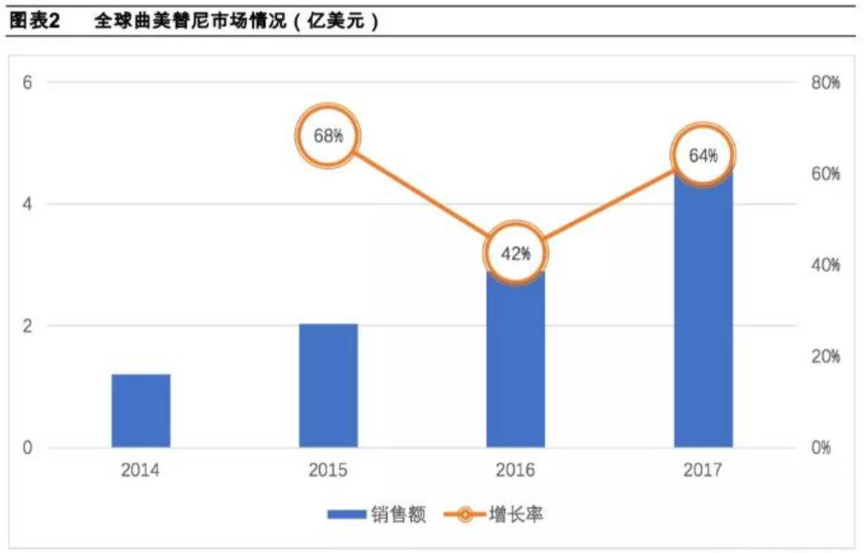
According to the Comprehensive Drug Database (PDB), Qumetinib has maintained rapid growth in 2014-2017. Global sales in 2017 were nearly US$500 million. After a large fluctuation in 2016, the growth rate resumed in 2017. A 64% high growth rate.
   (Source: Medical Geography)
In total, the two new drugs have a market share of nearly 1.1 billion US dollars (about 7.3 billion) in the world, and still maintain a low growth rate, and there may be a larger market prospect in China.
â– Domestic meditation, the third is coming? !
According to the data, Cinda submitted the application for the listing of adalimumab analogues, which is the biosimilar drug of Xiulele. The progress ranks third in the country. The first two are Baiaotai and Hisun Pharmaceutical.
The original research drug Xia Mei Le (adalimumab injection) is the world's first approved monoclonal antibody against human tumor necrosis factor, which has been approved worldwide for up to 14 indications. The name.
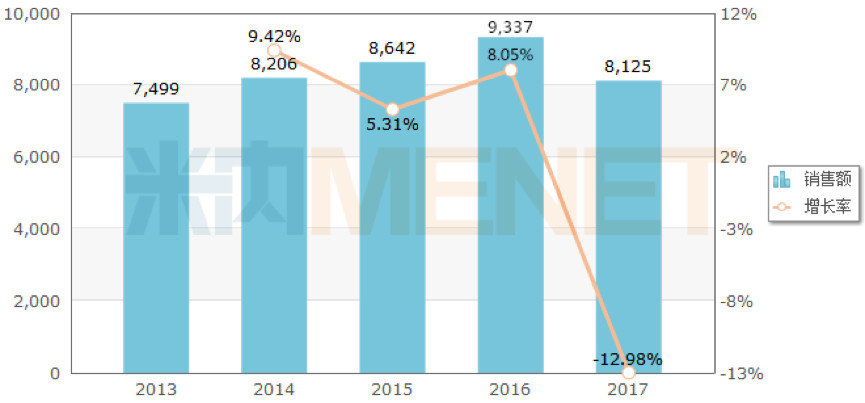
However, it is clear that China does not buy the title of “King of Medicineâ€. According to the data of the competition pattern of the Chinese public medical institutions in the intranet, the sales of adalimumab in 2017 is 81.25 million, which is far lower than the target drug Yisaipu ( Sales of recombinant human type II tumor necrosis factor receptor antibody fusion protein for injection).
  (Source: Minenet)
â–æ£å¤§å¤©æ™´, Hengrui, Haosen new drug acceptance number TOP3
China's market environment is complex, and drug research and development is susceptible to many factors such as technology, approval, and policies. There are many uncertain factors. To reform the drug review and approval system and solve the backlog of drug review. On February 26, 2016, the former State Food and Drug Administration issued the “Opinions on Resolving the Backlog of Drug Registration Applications for Priority Review and Approvalâ€.
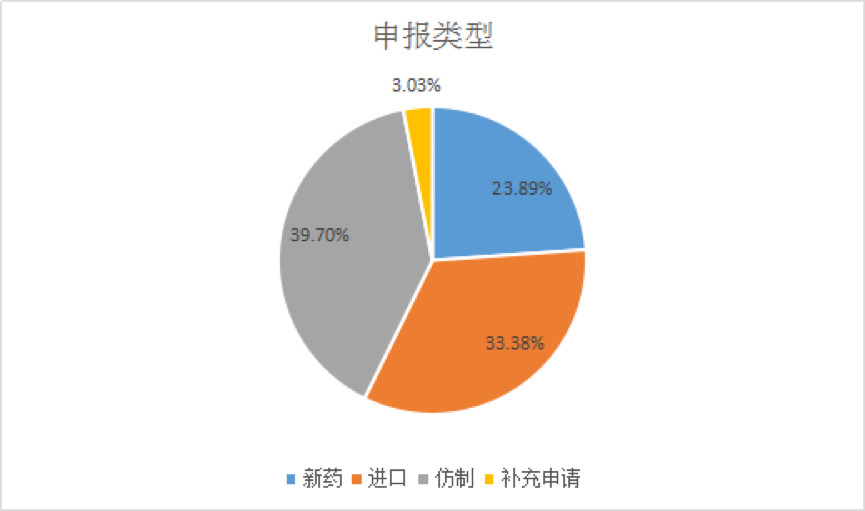
Therefore, 2016 is a key year for priority review and approval. According to the drug network, as of February 23, 2019, the number of drugs accepted for priority review reached 791. Among them, the proportion of generic drugs reported was the highest, at 39.70%, followed by new drugs, accounting for about one-third.
(Source: Yaozhi)
According to the type of application, it can be divided into three categories: new drugs, generic drugs, and imported drugs.
Among them, the reasons for the new drug are major special items, innovative drugs with obvious clinical value, and new drugs with obvious therapeutic advantages. According to the statistics of Yaozhi.com, the total number of new drug acceptance numbers included in the priority review is 189.
Among the companies that applied for new drugs, Zhengda Tianqing had the largest number of acceptances, with 17, followed by Hengrui Medicine and Haosen Pharmaceutical, with 12 and 8 respectively.
(Source: Yaozhi)
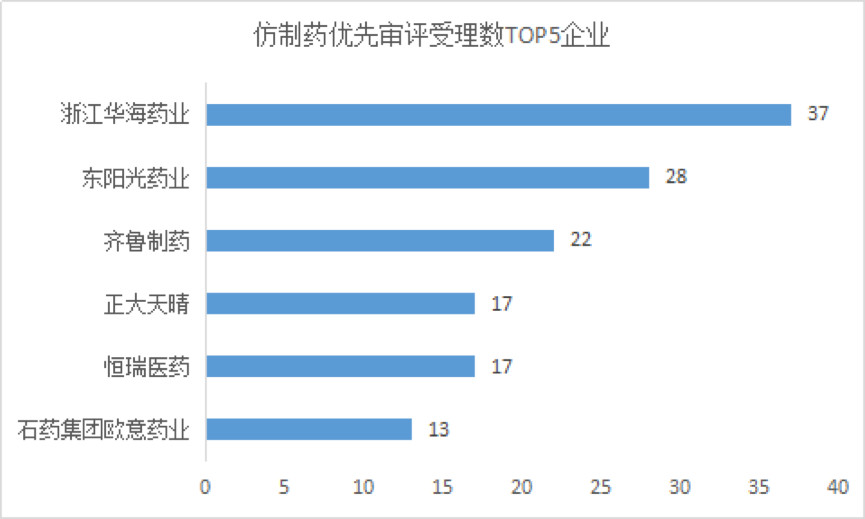
The generic drugs that have the first imitation products, the children's drugs that contribute to the products, and the drugs with obvious therapeutic advantages compared with the existing treatment methods will be included in the priority review and approval. The current generic drug acceptance number is 315, Zhejiang Huahai, Dongyang Sunshine Pharmaceutical and Qilu Pharmaceutical are the top companies for reporting enterprises .
(Source: Yaozhi)
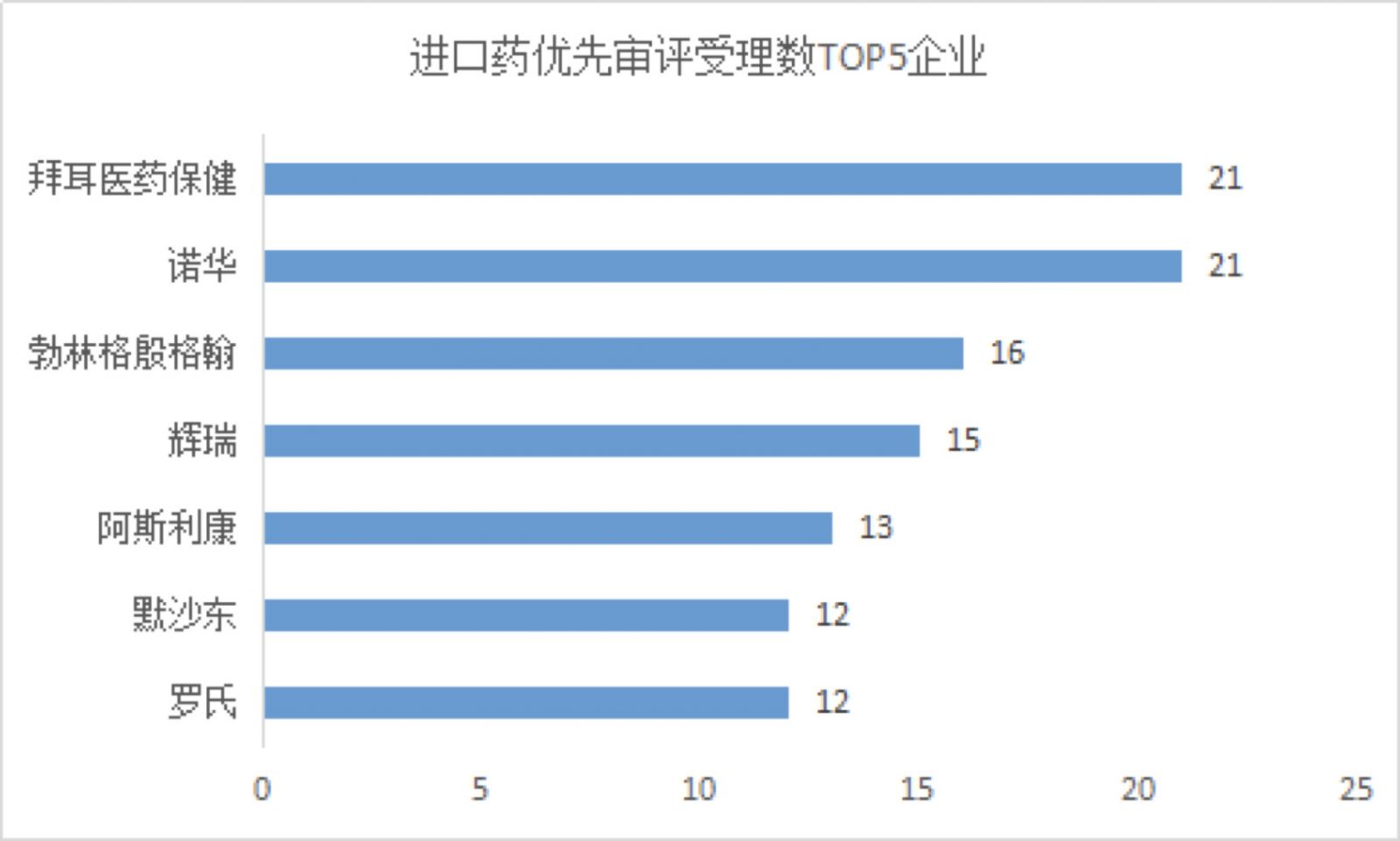
Among the reasons for the declaration of imported drugs, they are mostly oncology drugs, clinically urgently needed drugs, and rare diseases. The number of applications for importing medicines reached 263, and the top companies of the applicants were Bayer, Novartis and Boehringer Ingelheim.
(Source: Yaozhi)
