Commercial Gym Fitness Equipment combine innovative patented technology with total entertainment options to provide an exceptional user experience. The design of multiple preset programs enables a variety of treadmill exercises. Household Treadmill,Commercial Gym Fitness Equipment,Touch Screen Fitness Training,Training Fitness Equipment Haloxylon Ammodendron Medical Equipment Co., Ltd. , https://www.ssmedicaldevic.com
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot.
Sterile by EO conformity with ISO11137 by a CE marked sterilize center.
WEGO-Polyester Features
Structure
Polyfilament, braided and coated
Chemical Composition
Polyethylene terephthalate
Coating
Silicone or Uncoated
Color
Green or White
Sizes
USP 3- USP 6/0 metric6 - metric 0.7
Mass absorption
Non-absorbable
Indications
Skin Closure, Cardiovascular surgery, Vascular surgery, Ophthalmology, Orthopaedics, Gastrointestinal surgery, Neurosurgery
Contraindications
Not Known
Sterilization
Ethylene oxide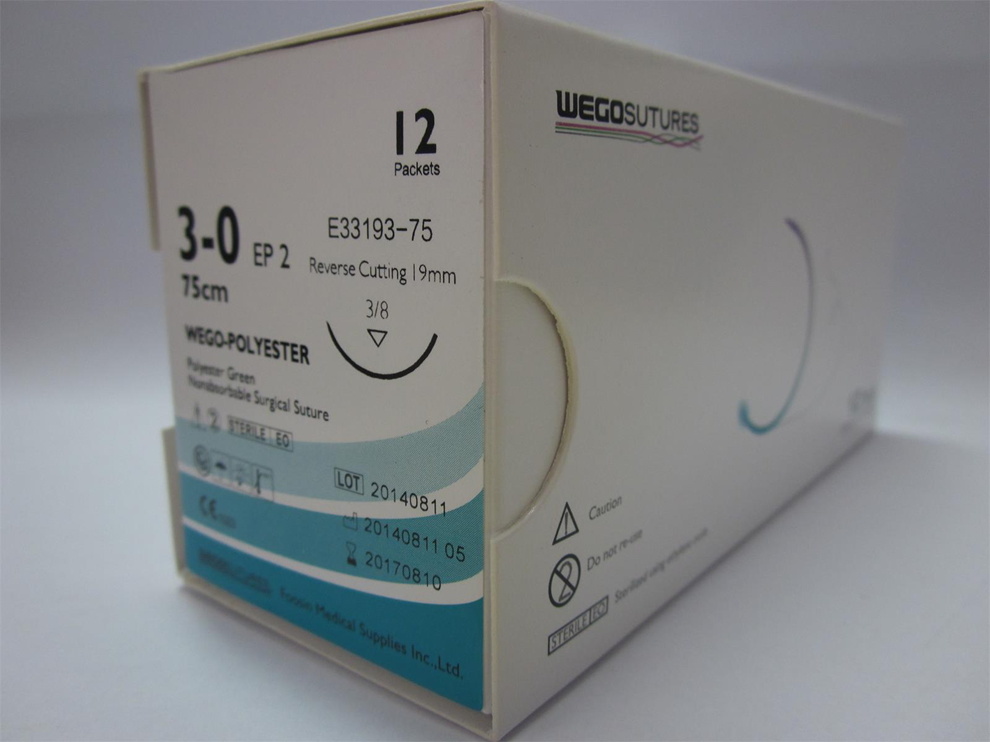 WEGO HOLDING COMPANY LIMITED ("WEGO") established in March 1988, and the major business of WEGO focus on medical devices and pharmaceuticals. In addition, WEGO develops some other business such as real estate, investment and so on. WEGO has 9 industry groups and more than 30 subsidiaries. The main products of WEGO are infusion set& syringe, blood transfusion equipment, heart stent & intracardiac consumable,IV catheter & special needle, blood purification equipment and consumables, orthopedic material, operation room equipment and accessories, wound care product, minimally invasive instrument and equipment, ICU product and accessories, high-capacity injection and pharmaceutical, renal product, biological diagnostic reagent, PVC & non-PVC material and so on. WEGO produces more than 30 series, 400 categories and 40,000 specifications products, and has become one of the most reliable suppliers of medical system in the word. At the same time, WEGO energetically takes part in healthcare service and provides renal dialysis service. WEGO's holding subsidiary ---SHANDONG WEIGAO GROUP MEDICAL POLYMER CO., LIMITED is listed in Hong Kong.
WEGO HOLDING COMPANY LIMITED ("WEGO") established in March 1988, and the major business of WEGO focus on medical devices and pharmaceuticals. In addition, WEGO develops some other business such as real estate, investment and so on. WEGO has 9 industry groups and more than 30 subsidiaries. The main products of WEGO are infusion set& syringe, blood transfusion equipment, heart stent & intracardiac consumable,IV catheter & special needle, blood purification equipment and consumables, orthopedic material, operation room equipment and accessories, wound care product, minimally invasive instrument and equipment, ICU product and accessories, high-capacity injection and pharmaceutical, renal product, biological diagnostic reagent, PVC & non-PVC material and so on. WEGO produces more than 30 series, 400 categories and 40,000 specifications products, and has become one of the most reliable suppliers of medical system in the word. At the same time, WEGO energetically takes part in healthcare service and provides renal dialysis service. WEGO's holding subsidiary ---SHANDONG WEIGAO GROUP MEDICAL POLYMER CO., LIMITED is listed in Hong Kong.
CE and FDA approved Absorbable sutures by full US and UK material. WEGOSUTURES conformity with the USP and EP standard. WEGOSUTURE is soft and easy for safe knot.
Sterile by EO conformity with ISO11137 by a CE marked sterilize center.
WEGO-Polyester Features
Structure
Polyfilament, braided and coated
Chemical Composition
Polyethylene terephthalate
Coating
Silicone or Uncoated
Color
Green or White
Sizes
USP 3- USP 6/0 metric6 - metric 0.7
Mass absorption
Non-absorbable
Indications
Skin Closure, Cardiovascular surgery, Vascular surgery, Ophthalmology, Orthopaedics, Gastrointestinal surgery, Neurosurgery
Contraindications
Not Known
Sterilization
Ethylene oxide
Equipped with IFT driver controller(Touch Screen Fitness Training)
IFT drive controller supports 4.0hp AC induction motor and integrated Footplant TM technology to control the speed belt, bringing users a smooth and natural feeling during running or walking. lGround Effects® The control technology
The unique suspension system, which is easy to wear on the knees, legs and back, reduces impact and controls lateral movement while remaining responsive.
Fully integrated entertainment options(Training Fitness Equipment)
The Personal visual screen of the Cardio Theater's overall entertainment design creates a one-of-a-kind high-end aerobic gym and enhances your ultimate membership experience.
Sterile Polyester Non-Absorbable Surgical Suture
Model NO.: Polyester
Logo Printing: Without Logo Printing
Medical Devices Ad. Approval No.: Type3
Medical Device Regulatory Type: Type 3
Package: Foil Bag
Box: 12 Pieces/Box
Carton: 100 Boxes/Carton
Trademark: Wegosuture or OEM brand
Transport Package: Aluminum Pack and Blister Pouch With Sterilize Ind
Specification: Ep, Usp
Origin: China
HS Code: 9018322000
Model NO.: Polyester
Logo Printing: Without Logo Printing
Medical Devices Ad. Approval No.: Type3
Medical Device Regulatory Type: Type 3
Package: Foil Bag
Box: 12 Pieces/Box
Carton: 100 Boxes/Carton
Trademark: Wegosuture or OEM brand
Transport Package: Aluminum Pack and Blister Pouch With Sterilize Ind
Specification: Ep, Usp
Origin: China
HS Code: 9018322000
WEGO HOLDING COMPANY LIMITED ("WEGO") established in March 1988, and the major business of WEGO focus on medical devices and pharmaceuticals. In addition, WEGO develops some other business such as real estate, investment and so on. WEGO has 9 industry groups and more than 30 subsidiaries. The main products of WEGO are infusion set& syringe, blood transfusion equipment, heart stent & intracardiac consumable,IV catheter & special needle, blood purification equipment and consumables, orthopedic material, operation room equipment and accessories, wound care product, minimally invasive instrument and equipment, ICU product and accessories, high-capacity injection and pharmaceutical, renal product, biological diagnostic reagent, PVC & non-PVC material and so on. WEGO produces more than 30 series, 400 categories and 40,000 specifications products, and has become one of the most reliable suppliers of medical system in the word. At the same time, WEGO energetically takes part in healthcare service and provides renal dialysis service. WEGO's holding subsidiary ---SHANDONG WEIGAO GROUP MEDICAL POLYMER CO., LIMITED is listed in Hong Kong.