First, the application background A carbonated beverage (soda) product is a beverage that is filled with carbon dioxide gas under certain conditions. Carbonated drinks, the main components include: carbonated water, citric acid and other acidic substances, white sugar, spices, and some contain caffeine, artificial colors and so on. In addition to sugars that can replenish energy to the body, inflated "carbonated drinks" contain almost no nutrients, and excessive consumption is harmful to the body. Reducing sugar refers to a reducing sugar capable of reducing a H. von Fehling reagent (Benedict reagent) or a B. Tollens reagent. Among the saccharides, a monosaccharide having a free aldehyde group or a ketone group in the molecule and a disaccharide containing a free aldehyde group are all reducible. Reducing sugars include glucose, fructose, galactose, lactose, maltose, and the like. We use potentiometric titration to determine the content of reducing sugar in carbonated beverages. The method has high accuracy and simple and convenient operation. According to the standard: GB/T 5009.7-2003 National Food Safety Standard Determination of reducing sugar in food Second, supporting instruments and reagents Instrument: Thunder magnetic ZDJ series titrator (potentiometric titration) Electrode: 213 platinum electrode, 232-01 calomel reference electrode Reagents: 0.5mol/L potassium permanganate standard titration solution (preparation and calibration reference document M9105-2015) Alkaline copper tartrate solution: Weigh 34.639g copper sulfate (CuSO4•5H2O), add appropriate amount of water to dissolve, add 0.5mL sulfuric acid, and then dilute to 500mL with water. Alkaline copper tartrate solution: Weigh 34.5g of sodium potassium tartrate and 50g of sodium hydroxide, add appropriate amount of water to dissolve, add water to dilute to 500mL, and store in rubber stopper glass bottle. Ferric sulfate solution: Weigh 50g of ferric sulfate, add 200mL of water to dissolve, slowly add 100mL of sulfuric acid, and then dilute to 1000mL after cooling. Third, the experimental process 1. Pipette 100.0mL of the sample into a beaker, remove the carbon dioxide on the water bath, transfer it to a 250mL volumetric flask, and wash the beaker with water. The washing solution is added to the volumetric flask, and then added to the scale, mixed, and set aside. Pipette 1.00 mL of the treated sample, add 25 mL of alkaline copper tartrate solution and 25 mL of Ethyl solution in a 500 mL beaker, cover a beaker on the beaker, heat, and control to boil within 4 min. Boil for 2 min, filter while hot, and wash the beaker and precipitate with hot water until the wash is not alkaline. Place the brick red filter residue into the titration cup, add 25 mL of ferric sulfate solution and 25 mL of water, and stir with a glass rod to completely dissolve the cuprous oxide. Place on the automatic titrator. At the same time, do a blank test. 2, instrument operation a) Place the electrode holder above the titration cell and place the measuring electrode, reference electrode and filling tube. b) Turn on the instrument, connect the 213 platinum electrode to the measuring electrode interface, and connect the 232-01 calomel reference electrode to the reference electrode interface. c) Connect the liquid take-up tube to the potassium permanganate standard titration solution, use the instrument to clean the button, clean it three times, and connect the cleaning solution to the waste cup. d) Click Start Method Titration, select Dynamic Titration, and set the parameters. e) Click to start the measurement and wait for the instrument to automatically detect. f) At the end of the analysis, the instrument displays the conclusion and automatically saves the map and data. g) If it is no longer measured, use the cleaning button, connect the infusion tube to pure water, and wash it three times. h) Turn off the instrument, soak the reference electrode in saturated potassium chloride, and measure the electrode dry. Fourth, the calculation formula X=71.54× c(V 1 -V 0 ) Where: X—the mass of the reducing sugar in the sample is equivalent to the mass of cuprous oxide, and the unit is milligram (mg); V1—the value of the volume of potassium permanganate standard titration solution consumed in titration of the sample, in milliliters (mL); V0—the value of the volume of potassium permanganate standard titration solution consumed when titrating the blank, in milliliters (mL); c—The actual concentration of the potassium titanate standard titration solution, in moles per liter (mol/L). among them: X—the value of the reducing sugar content in the sample, in grams per hundred grams (g/100g); M1—the value of the mass of the reducing sugar obtained by looking up the table, in milligrams (mg); M2—The value of the sample volume in milliliters (mL). Five, matters needing attention 1) The electrode should be immersed in distilled water, protein solution and acidic fluoride solution for a long time. 2) The filling liquid of the reference electrode is 3mol/L potassium chloride solution. The filling liquid can be added from the small hole at the upper end of the electrode. When not used for a long time, the liquid filling hole is plugged and a protective sleeve is installed to prevent the filling liquid from drying out. JRT usb interface distance sensor board adds a USB port to a Laser Distance Sensor, inexpensive laser distance measurement sensors, allowing customers directly using GBeacon-2.3-Fx.exe software to control the rangefinder sensor. Usb laser distance sensors are the the most popular model devices, it works by measuring the phase of an visible-red laser beam that reflects on the target. Usually, customers would like to choose CH341SER.EXE version to test. USB Laser Distance Sensor,Distance Sensor,Laser Distance Sensor,Distance Measurement Sensors Chengdu JRT Meter Technology Co., Ltd , https://www.accuracysensor.com
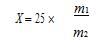
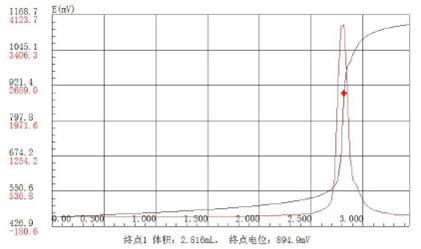
Overall scheme for detecting reducing sugar content in carbonated beverages
