In a recent blog post, I explored how to use critical thinking to understand the application and research of artificial intelligence in the medical field, and to focus research on the correlation between these artificial intelligence experiments and clinical applications. But after that, I think of a problem, that is, some studies have made progress, and some studies are far from reaching the stage of clinical application. There is no simple and clear way to discuss this process. People who are engaged in medical research may agree with this view because the medical community has solved this problem. In fact, in the medical field, according to the experimental results will have a great effect on clinical applications, clinical trials can be divided into three categories. These categories are called different stages of medical research in the industry and reflect the usual path from pre-production to clinical application. These categories are precisely the only way for clinical innovation to be accepted by doctors and regulatory agencies. In general, most human-related medical research is divided into these three categories (or three stages). The first phase is the first safety check. Initially, the drug needs to be tested on a small group of subjects to ensure that there are no terrible consequences. At this stage, we rarely even consider whether the drug being tested has efficacy (ie, what happens after the subject takes the drug), and only wants to confirm that the test does not cause the patient to die. If we get good feedback from it, it shows that the drug does work well, it is good, but it is not the main goal and motivation of the trial. The second phase will provide a more comprehensive assessment of test safety. At this stage, it is necessary to expand the size of the test population. This is to find out whether the drug has rare side effects. Because of the larger number of test samples, more evidence of drug-related evidence may be found, but even so, this phase is never sufficient to justify clinical application. The third phase is the most costly, difficult, but important phase. The main goal is to find out how much the drug can play, which usually means that a large number of subjects must try a certain drug for a long time, and the methods and analysis used in the test can withstand the United States. Strict review by the Food and Drug Administration (FDA) or similar government regulatory authorities. In addition, from a technical point of view, there are actually preclinical trials (animal experimental models) and phase IV clinical trials (subsequent trials after the introduction of new drugs). However, the above three stages are the processes that must be experienced to turn a medical idea into an actual therapeutic drug. I believe that the advancement of medical artificial intelligence research is very similar to the routine medical clinical trial process, because almost all medical artificial intelligence research I have witnessed can be classified or clearly defined in the three known stages. As for whether this system is very strict, whether it covers enough cutting-edge research, I can't give a 100% positive answer, but this article can at least form a practical idea when designing or understanding medical artificial intelligence research. The framework provides some help. The best choice for Analog hearing aids with earbuds design The best choice for Analog hearing aids with earbuds design Shenzhen Sunshine Technology Co.,Ltd , https://www.szyatwin.com
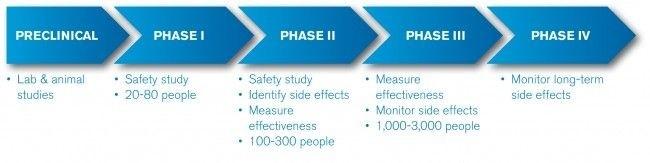
Innovating medical care with AI must go through these three stages
